2.10 Surficial Mineral Systems
This content is being archived
Please be informed that this content is in the process of being archived. Please see our Legacy Publications page for further information.
Surficial systems result from the physical and chemical phenomena which cause concentration of ore material within the regolith, lake, or shallow marine environment, generally by the action of the environment. This occurs as a result of the following processes:
- Supergene enrichment, i.e. weathering via oxidation, biological and/or chemical attack of a rock, either liberating rock fragments or creating chemically deposited minerals, clays, or laterites;
- Deposition by sedimentary processes, including erosion, winnowing, and density separation; and
- Deposition in low-energy environments such as beach and marine environments and terminal lakes.
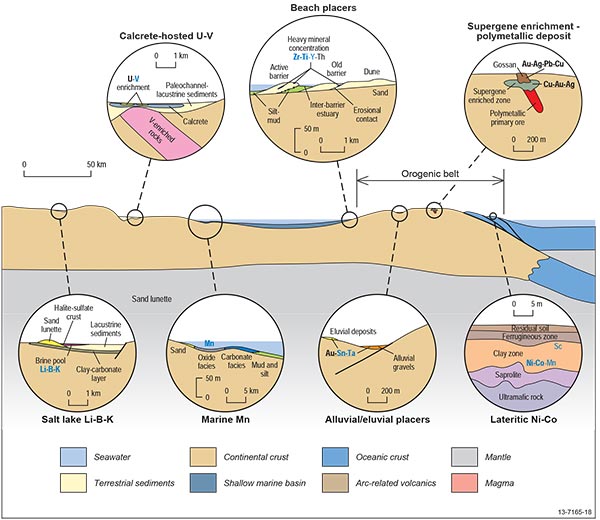
Figure 2.10.1: Diagrammatic sketch of the surficial mineral system illustrating the relative location of deposits types within the overall setting and the likely distribution of critical and other commodities within and around these deposit types. In the commodity lists, blue indicates critical commodities, underlined bold indicates major products, bold indicates commonly recovered by-products, underlined normal font indicates commodities with limited recovery as a by-product (usually during downstream processing), and normal text indicates commodities that are geochemically anomalous, but not recovered.
Due to the different depositional environments we have divided surficial systems into three main types: (i) those formed by supergene enrichment process, (ii) placer deposits formed by density separation during sedimentary processes, and (iii) salt lakes and calcrete uranium systems (Figure 2.10.1).
2.10.1 Supergene enrichment
2.10.1.1 Geological setting
Lateritic Ni-Co deposits are associated with ophiolites and mafic/ultramafic complexes in mobile belts and Archaean/Proterozoic greenstone terranes. Uplift is required to expose these rocks to weathering.
Lateritic P-REE deposits are associated with carbonatites and alkaline igneous complexes. Carbonatites occur in cratons while alkaline igneous rocks may occur in continental rift valleys, intraplate magmatic provinces and at destructive plate boundaries as discussed above.
Primary Cu, Pb and Zn deposits form in a number of tectonic settings as described above. Secondary mineralisation occurs when the primary sulfide minerals are oxidised at the surface and acidified meteoric water carries the Cu and Zn down to the water table where Cu is commonly deposited. Lead and Ag are commonly retained in the weathered profile as they are insoluble in most surficial conditions. Zinc is generally lost during weathering, although under some conditions, it can be deposited to form non-sulfide Zn deposits. Bauxite requires intense weathering conditions and is best developed in tropical climates.
Manganese deposits generally form in shallow marine depositional environments (15–300 m), commonly in sheltered sites around islands along some areas of continental shelf and the interior basins. Most deposits overlie oxidised substrates, but basinward, carbonate deposits may be in reducing environments. Many deposits are in within transgressive stratigraphic sequences near or at black shale pinchouts.
2.10.1.2 Sources of fluid, metals and energy
Supergene processes involve predominantly meteoric water circulation. The descending meteoric waters oxidise the primary sulfide and other minerals and redistribute ore elements. The phenomenon is most common in arid or semi-arid regions. The sources of metals are the protoliths that are being weathered. For example, ultramafic and mafic rocks are the sources of Ni, Co, Cr, Sc and PGE in lateritic deposits, whereas pre-existing hypogene ores are the sources of supergene Cu, and carbonatites can be sources of light REE, U, Th, Sr, Ba, Nb, Ta, Pb, Zn, V and P. Bauxite can form from any aluminous silicate rock and Mn is sourced from seawater.
2.10.1.3 Fluid pathways
Meteoric waters flow in the weathering profile under moderate topography with concomitant oxidation and chemical weathering of pre-existing ore minerals.
2.10.1.4 Depositional processes
Lateritic Ni-Co, lateritic P-REE and secondary Cu, Pb and Zn deposits are the result of supergene processes. Supergene enrichment occurs at or near the base of the weathering profile developed over hypogene sulfide deposits or rocks enriched in Ni and Co. Metals that have been leached from the oxidised part of the profile are carried downward by percolating groundwater, where they are deposited either by a change in redox as the water table is reached or through reactions with hypogene sulfide minerals. These reactions produce secondary oxides and/or sulfides with metal contents higher than those of the primary ore or protolith.
Bauxite formation requires climates that provide relatively high rates of chemical weathering and relatively low rates of physical erosion. In this case Al enrichment is residual and involves removal of other constituents of the rocks, leaving a residua composed of bauxite.
Manganese precipitation is believed to take place in stratified water masses at the interface between anoxic seawater and near surface oxygenated waters. Extreme Fe fractionation is caused by a low solubility of Fe in low Eh environments where Fe precipitates as iron sulfide. A subsequent increase in Eh and/or pH of Mn-rich water may produce Mn-rich, Fe-depleted chemical sediments. The Mn oxide facies is preserved on oxidised substrates. The Mn ore is commonly further enriched by supergene process.
2.10.1.5 Australian examples
The Murrin Murrin lateritic Ni-Co deposit developed from thoroughly serpentinised peridotite in the Late Archean Norseman-Wiluna greenstone belt in Western Australia (Monti and Fazakerley, 1996; Wells and Butt, 2006). At Murrin Murrin the laterite profile consists of five zones, including: unweathered country rock at the base, saprolite, smectite, limonite, and a cap of colluvium. The highest Ni concentrations occur in the smectite zone. The cobalt occurs in Mn-oxides in the limonite zone and upper part of the smectite zone.
The SCONI project in Queensland includes five key lateritic Ni-Co deposits, with the Greenvale deposit being an historic mine. Recent exploration in this area by Metallica Resources indicates that this region also contains significant reserves of Sc. Other lateritic Ni-Co deposits include Wingella and the Kalgoorlie Nickel Project in Western Australia and the Barnes Hill project in Tasmania.
Mount Weld is the only currently operating lateritic phosphate-REE mine in Australia. It contains some of the highest REE grades (8.1% Rare-Earth Oxides) for this style of deposit making it a world-class deposit. Mount Weld also hosts significant resources of Nb, Ta, and P2O5. The Crown deposit in the northern part of the complex has an indicated and inferred resource of 37.7 Mt at 1.07% Nb2O5, 1.16% total lanthanide oxides, 0.09% Y2O3, 0.3% ZrO, 0.024% Ta2O5, and 7.99% P2O5 (as of January, 2010), while P mineralisation in the Swan deposit in the northern half of the complex has an indicated and inferred resource of 77 Mt at 13.5% P2O5. The Anchor deposit in the southwest of the Mount Weld complex contains Nb-Ta mineralisation (Hoatson et al., 2011).
Bauxite is currently mined at Weipa (Qld), Gove (NT) and the Darling Range (WA), but other areas of potential include the Mitchell Plateau and Cape Bougainville (WA), Cape York (Qld), central New South Wales and parts of Tasmania.
The Groote Eylandt Mn mine in the Gulf of Carpentaria accounts for about one quarter of the world's total Mn production. The mine had proven, probable reserves of 109 Mt grading 46.6 % Mn for 50.794 Mt of manganese at June 30, 2010 (Miningoilgas, 2013). Mn is also being mined at Bootu Creek in the Northern Territory and Woodie Woodie in Western Australia (Geoscience Australia, 2012).
2.10.1.6 Associated critical commodities
The limonite in lateritic Ni-Co deposits commonly contains other elements such as Ca, Zn, Cu, Mn, Cr and, depending on the separation of the lateritic layers, Mg as well. All of these elements are commonly present as oxides and hydroxides. As mentioned above, Sc is also associated with lateritic Ni-Co deposits in north Queensland and Metallica Minerals Limited has announced a total (measured + indicated + inferred) Sc resource of 3827 tonnes for its combined SCONI southern deposits (Metallica Minerals Ltd., 2012). Other deposits are known near Young and Nyngan in New South Wales.
At Mount Weld, the lateritic phosphate-REE zone contains abundant insoluble phosphates, aluminophosphates, clays, crandallite-group minerals, Fe and Mn-bearing oxides that contain elevated concentrations of REE, Y, U, Th, Nb, Ta, Zr, Ti, V, Cr, Ba, and Sr. Very high-grade lanthanide concentrations (up to 45% combined lanthanide oxides) occur in the regolith and are attributed to secondary monazite. Churchite contains considerable amounts of high-grade Y (up to 2.5% Y2O3). Niobium- and Ta-bearing pyrochlore, ilmenite, and rutile in the primary carbonatite are concentrated in the apatite and magnetite-rich residual zone. Grades are typically variable and locally high (up to 1.5% Nb2O5 and 0.05% Ta2O5). Higher grades of niobium (up to 6% Nb2O5) occur in the supergene zone.
Significant reserves of Ga occur in oxide minerals derived from surficial weathering of polymetallic ore deposits (U.S. Geological Survey, 2013). Other trace elements include Ge, Se, Ag, Cd, and Sb.
More than 30 different trace elements occur in bauxite and they range widely in abundance. Bauxite residues may contain up to 9% TiO2 and analysis of bauxite from Weipa (Jepsen and Schellmann, 1974) shows these ores contain 73 ppm Ce, 120 ppm Cr, 67 ppm Ga, 37 ppm La, 198 ppm P, 38 ppm Th, 490 ppm V, 37 ppm Y and 966 ppm Zr. A Russian patent has demonstrated that a resin-in-pulp method can be used for the recovery of Ti, Sc, U and Th from Australian bauxite residues (Klauber et al., 2009), and processes for recovering REE and Sc are being developed in the Philipines and Indonesia.
A wide range of trace elements have been reported (Parcejus and Bolton, 1992) from the Mn oxide ores at Groote Eylandt, including B, As, P, Cr, Co, Cu, Mo, Ti, U, Th, Zr, V, Zn, Pb, Sc, Yb, Ce, Gd, Eu, La, Nd, P, and Sm. Parcejus and Bolton (1992) suggest that some of these elements are correlated with certain minerals. For example, they suggest that Ti and Zr are correlated with kaolinite, whereas Gd is correlated with goethite, and Pb with gibbsite.
2.10.2 Placer Deposits
2.10.2.1 Geological setting
Diamond placers most commonly form in stable cratonic settings. Tin placers form in Paleozoic to Cenozoic accreted terranes or stable cratonic foldbelts that contain highly evolved granitoid plutons or their extrusive equivalents. Channel iron deposits usually occupy meandering palaeochannels in the Early to Mid-Tertiary palaeosurfaces of Western Australia. Gold placers may form in Cenozic conglomerates along major fault zones, in cratonic areas where erosion has proceeded for a long time producing multicycle sediments, and in high-level terrace gravels.
Heavy mineral sand deposits containing rutile (TiO2), ilmenite (FeTiO3), zircon (ZrSiO4) and monazite ([Ce,La,Th,Nd,Y]PO4) occur on the margins of cratons. They usually form in beach environments and require crustal stability during deposition and preservation of the deposits.
2.10.2.2 Sources of fluid, metals and energy
Diamonds are sourced from kimberlite pipes but alluvial diamond deposits may be up to 1000 km from the source. The source of iron for channel iron deposits is thought to be iron-rich soils which developed upon a palaeosurface during hot, humid conditions. Placer Au is usually sourced from pre-existing hydrothermal gold deposits which are eroded by rivers and more rarely glaciers. Tin is sourced from highly evolved granitic plutons or their extrusive equivalents.
Heavy mineral sand deposits come from granitic and volcanic rocks within the erosional areas of rivers which carry the sediment into the ocean, where it is caught up in littoral drift or longshore drift near beaches.
2.10.2.3 Fluid pathways
Glacial and fluvial processes provide a mix of depositional environments including high-energy bench (or terrace) gravels and lower-energy deposits on the valley floor. Near-shore (beach) deposits result from the efficient sorting and winnowing action of the surf. Deposits in arid climates may form by the winnowing action of the wind.
2.10.2.4 Depositional processes
Alluvial placers are formed by the deposition of dense particles at a site where water velocity remains below that required for further transport. Tin placers form where stream gradients lie within the critical range for deposition of cassiterite (for instance, where stream velocity is sufficient to result in good gravity separation but not enough so the channel is swept clean). Diamond and Au placers form on the inside bends of rivers and creeks; in natural hollows; at the break of slope on a stream; and at the base of an escarpment, waterfall or other barrier. Channel iron deposits are formed by the erosion of an iron-rich palaeosurface into palaeodrainage systems, where the Fe becomes consolidated within the existing river courses.
Heavy mineral sand deposits essentially fall into two categories depending on the mode of deposition: alluvial or aeolian. Alluvial deposits are further split into marine beach placers (or strandlines) and lacustrine heavy mineral accumulations. Aeolian deposits are generally closely associated with marine beach placers, having been formed by the erosion, transport and deposition of heavy minerals from adjacent marine beach placers by prevailing winds.
2.10.2.5 Australian examples
Discovery of alluvial diamonds at Smoke Creek in Western Australia in the late 1970s led to the discovery of the currently mined Argyle diamond pipe. Thus, the Smoke Creek alluvial diamonds are most likely derived from erosion of the Argyle pipe, and therefore represent a possible new source of valuable pink diamonds. North Australian Diamonds Limited is also examining the diamond-bearing potential of gravels near Borroloola in the Northern Territory (Geoscience Australia, 2012).
Tin placer deposits include the Karaula alluvial-tin-tungsten deposit near Inverell, and the the Giants Den alluvial deposits near Bendemeer in New South Wales. In Tasmania exploration for alluvial Sn is continuing at Scotia, Endurance and in Ringarooma Bay (Geoscience Australia, 2012).
Channel iron deposits are only known to occur in Western Australia and Kazahkstan. Australian examples include the Yandicoogina South deposit and the Blacksmith and Anvil tenements in the Pilbara region.
Alluvial Au deposits were historically mined in the major goldfields in Victoria, New South Wales, Western Australia, Queensland and Tasmania. In Western Australia, current alluvial Au projects include Ivanhoe Yalgoo, Mount Sholl East, Nemisis, Nicholsons Find, Sharks Gully and Womerina (not shown in Figure 2.1.1). Until recently, alluvial Au was being mined from the Georgetown gold mine in Queensland (ERO Mining, 2010). Queensland Bauxite Limited has also identified an alluvial Au system near Tamworth, New South Wales.
Heavy mineral sand deposits represent a major source of rutile, ilmenite, zircon, and in the past, monazite. Australian examples include Enabba, Cooljaroo, Dardanup, Gwindinup and Calypso (Western Australia), Jacinth-Ambrosia and Cyclone (South Australia), Douglas and the various WIM deposits (in the Murray Basin, Victoria), Ginko and Snapper (New South Wales), and North Stradbroke Island (Queensland). The latest emerging heavy mineral sand province is the Eucla Basin in South Australia where zircon is the dominant commercial mineral rather than the titanium-rich minerals.
2.10.2.6 Associated critical commodities
Diamond placers are commonly associated with Cr, Ti, Mn, Ni, Nb Co, PGE, and Ba, some of which are used to indicate the proximity to diamond deposits. Tin placers are associated with anomalous amounts of As, B, F, W, Be, Cu, Pb, Zn, Mn, Nb, Ti, and Y (Ogwuegbu et al., 2011). Gold placer deposits have anomalous amounts of Ag, As, Hg, Sb, Cu, Fe, W, Ti, Zr, S and Cr. Heavy mineral sand deposits have associated REE, Cr, Sn, Th and U.
2.10.3 Salt Lakes (Li-K-B) and Calcrete Uranium
The following is based upon a preliminary assessment of the potential of Australian salt lakes to host Li, B and potash deposits. Readers are referred to the assessment (Mernagh, 2013) for a comprehensive review of Australian salt lake systems, and a preliminary assessment of their critical commodity potential.
2.10.3.1 Geological setting
The processes that lead to salt build-up may occur on the flanks of continental uplifts, within extensional basins, in endorheic basins, and in glaciated terrain. The majority of salt lakes are found in the semi-arid to arid regions of the world as evaporation in excess of precipitation plays a critical role in the development of salt lakes. Three basic conditions are needed to form salt lakes (Eugster and Hardie, 1978). First, outflow must be absent or severely restricted to ensure hydrological closure. Second, evaporation must exceed inflow, and thirdly, inflow must be sufficient to form a body of water at or very close to the surface. Therefore, favourable locations for the formation of salt lakes are arid basins in the rain-shadows of mountain ranges or highland areas, which provide the catchment for precipitation. In areas of lower relief, shallow basins may act as the focus of local discharge and evaporation from regionally extensive groundwater systems. Some of the world's largest salt lakes are found at elevations exceeding 1000 m, such as Salar de Uyuni (Bolivia), Salar de Atacama (Chile), Qinghai Lake (Tibet), and the Great Salt Lake (US). This reflects the tendency for these systems to form in tectonically active regions. Even some large saline lakes nearer to sea level (e.g. the Caspian and Dead Seas) are associated with active tectonism.
Calcrete uranium is associated with calcrete or dolocrete formed within Cenozoic drainage systems incised into rocks containing leachable U and V. Non-pedogenic calcrete (also known as groundwater or valley calcrete) is formed predominantly near the water table from groundwater moving along extremely low gradients. The formation of non-pedogenic calcrete is generally controlled by climate and the type of soil. In the Yilgarn Craton, in Western Australia, the distribution of non-pedogenic and pedogenic calcretes is defined by the Menzies Line. North of the Menzies Line, the zone dominated by non-pedogenic calcretes, the soils are generally neutral to acid and the groundwaters are less saline and neutral to alkaline. South of the Menzies Line, dominated by pedogenic calcretes, the soils are neutral to alkaline and the groundwaters are saline and neutral to acidic.
2.10.3.2 Sources of fluid, metals and energy
Climate plays a critical role in the water balance of salt lakes. The amount of inflow to a closed basin, including precipitation, must be closely balanced by evaporative loss in the basin in order to produce elevated salinities. The sources of fluids are mainly direct precipitation, associated surface flow, and/or groundwater. Groundwater may be derived from a local or regional meteoric system, interstitial water from sediments, or deep basinal or hydrothermal fluids. Hydrothermal discharge (e.g. from geothermal springs), though often small in volume can be significant in terms of its contribution of solutes.
Lithium can be sourced from volcanic glasses and felsic rocks, particularly andesitic to rhyolitic tuffs and ash flows. Another source is Li-bearing clays (e.g., hectorite) formed from the weathering of feldspars and micas. The occurrence of Li and B in salt lakes is also correlated with recent or concurrent volcanic activity and/or areas that have a higher than average geothermal gradient. Borate deposits, in particular, appear to be associated with long-lasting, high-B geothermal springs with a long-lasting volcanic period or a high underlying magmatic temperature (Garrett, 1998).
Sources of K are most commonly felsic to intermediate volcanic rocks, but also include older evaporites and continental sedimentary rocks (Alonso and Risacher, 1996; Risacher and Fritz, 2009). Sources of K in these rocks are weathered minerals, such as orthoclase, microcline, biotite, leucite, and nepheline. Studies have found a positive correlation between K, Li, and B in brines (Carmona et al., 2000; Orris, 1997; Zheng, 1984), which is probably indicative of the fact that many salt lakes occur in volcaniclastic terranes that typically are associated with convergent plate boundaries (Orris, 1997).
All known calcrete-hosted uranium deposits are located in palaeochannels incised into potential source rocks of K, U and V. Intensive weathering and erosion of felsic rocks can provide U as well as K to the calcrete system.
Mafic igneous rocks, sediments with V-rich clays, ironstone such as banded iron-formation and ferricrete are often enriched in V. Such rocks are generally present in the vicinity of calcrete-hosted uranium deposits and can provide the V needed for formation of the mineral carnotite [K(U+6O2)(V+5O4).xH2O] commonly associated with calcrete uranium deposits.
2.10.3.3 Fluid pathways
Most of the surface and groundwater is dominantly controlled by gravity-driven fluid flow. Gradients in potential energy (hydraulic head) drive fluid flow from regions of higher hydraulic head to regions of lower hydraulic head. Capillary forces may also affect fluid flow in the capillary fringe (a saturated zone above the water table). Geothermal gradients may lead to the discharge of hydrothermal fluids into the system. These higher temperature fluids not only add thermal energy to the system but potentially carry higher concentrations of solutes than other fluids.
The calcrete uranium system is driven by shallow groundwater drainage with extremely low gradients, ca. 10 m/km. The drainage in the region is controlled by a recharge area upstream and a system of playa lakes in the discharge area. In addition of the infill sediments, calcretes formed near the water table also function as aquifers which allow groundwaters to actively interact with the calcretes. The presence of playa lakes creates conditions where groundwaters in the palaeovalleys can mix with relatively more saline waters in the playas.
2.10.3.4 Depositional processes
The ultimate pathway of brine evolution of any salt lake may be associated with a few fundamental rock types, their mode of reaction with the dilute inflow waters, and the resulting relation of major cations and anions. By the time the surface and/or groundwaters have reached the periphery of the endorheic basin, water-rock reactions are superseded by other processes. From then on evaporative concentration normally plays the dominant role leading up to mineral precipitation but other factors (e.g., wetting and drying cycles and the precipitation of efflorescent crusts) may also influence brine evolution.
Carnotite is the principal U-bearing mineral in calcrete uranium deposits. The formation of carnotite in valley calcretes is closely related to the seasonal fluctuation of the groundwater table. The fluctuation is associated with evaporation of groundwaters which can lead to an increase in the concentration of dissolved K, V and U. It can also change the concentration carbonate ions in the water affecting the solubility of uranium. Evaporation is also important in the playa deposits where it can control the salinity of lake waters. The mixing of more saline lake waters, relatively enriched in K and Ca, and the incoming groundwaters from the drainage channel can be equally important in the formation of carnotite.
2.10.3.5 Australian examples
In Australia the salt lakes occupy features of the landscape that have changed little tectonically and have not been transgressed by seawater since at least the Paleogene (De Deckker, 1983). The playa lakes in Western Australia have been the sites of periodic lacustrine deposition since the mid-Miocene (Van de Graaff et al., 1977). Potash resources have been identified at Lake Dissappointment, Lake Chandler, Lake Mackay and the Dandaragan Greensands Project in Western Australia and at the Karinga Creek Project in the Northern Territory. Lakes that may be prospective for Li include Lake Tyrrell in Victoria, Lake Frome, Lake Eyre, Lake Torrens, Pernatty Lagoon, Lake Gilles and Lake Windabout in South Australia and Lake Austin, Lake Irwin and Lake Yindarlgooda in Western Australia.
The Yeelirrie calcrete uranium deposit in Western Australia is Australia's second largest undeveloped uranium deposit with a measured and indicated mineral resources of approximately 48 kt of U3O8, and an average grade of approximately 0.13% U3O8. Other calcrete uranium resources include Lake Way, Centipede, Lake Maitland and Thatcher Soak in Western Australia and the Napperby deposit in the Northern Territory (Geoscience Australia, 2013).
2.10.3.6 Associated critical commodities
The by-products of brine evaporation processes are all natural minerals, some of which are in common use. The major by-products include halite (NaCl), sylvinite (NaCl + KCl), carnallite (KMgCl3.6H2O), Li-carnallite (LiMgCl3.7H20) and bischofite (MgCl2.6H2O). However, the large algal mats commonly found on the edges of salt lakes have the potential to scavenge particular metals from the surrounding environment. Draper and Jensen (1976) have documented the close association between Mn and the presence of algal mats at Lake Frome.
Analysis of carnotite ore from Western Australia has shown that it also contains minor amounts of Na, Si, Ca, Mg, Al and Fe (Aral, 2010). Gangue minerals include quartz, kaolin, smectite, albite, calcite, dolomite, gypsum, barite and halite. The presence of gypsum, barite and halite indicate the prevelance of an arid environment.




