Uranium and thorium
Page last updated:1 September 2023
Introduction
Uranium and thorium are naturally occurring, radioactive heavy metals with unusual properties. The energy generated by the natural breakdown of radioactive elements is immense and can be used in nuclear reactors. Australia has significant resources of both uranium and thorium within the rocks that make up the Australian continent.
The mining of uranium has been a hot topic in Australia since the 1970s. Concerns about the safety of nuclear power and storage of nuclear waste, and a growing international stockpile of nuclear weapons, have sparked much discussion about Australia's role in supplying fuel for the nuclear industry. Australian governments have introduced laws and regulations that govern the mining of uranium and its sale to other countries that take into account international safeguards and seek to balance the interests of the environment, Indigenous Australians, regional communities and the mining industry.
Properties
Uranium and thorium are both silvery white-grey radioactive metals that corrode to black oxide in air. They are both malleable (can be pressed into shape) and ductile (can be beaten and drawn into a wire) and are very reactive and so cannot be found in the environment in their elemental forms. Thorium is much more abundant than uranium in rocks from the Earth's crust; it is found in small amounts in most rocks and soils. Uranium can form compounds with many of the metals; it reacts with hydrochloric and nitric acids, but other acids attack the element very slowly. Thorium is slowly attacked by water but, except for hydrochloric acid, does not dissolve readily in most common acids.
Uraninite or 'pitchblende' from Narbarlek, Northern Territory. R19144. Source: Geoscience Australia.
Uranium and thorium are not stable. They break down in a process called radioactive decay. More than 99% of natural uranium exists in a form (isotope) called uranium-238 while more than 99% of natural thorium exists as thorium-232. These metals decay very slowly eventually to form lead. During the decay processes, a series of new substances are formed including radium and radon, alpha and beta particles, and gamma radiation (see Google Arts and Culture: Hot Rocks).
While far less common, the uranium isotope uranium-235 is the only naturally occurring fissile material. This means it is able to undergo nuclear fission, a process used to create energy by the splitting of an atom nucleus. When a uranium-235 particle is bombarded with neutrons, most of the time, it divides into two smaller particles and releases nuclear energy and more neutrons. This is the fission process. These neutrons can then be absorbed by other uranium-235 particles, causing further fission, and a nuclear chain reaction occurs. During this process, large amounts of energy are released, typically in the form of heat.
As uranium-235 is so rare in nature, uranium has to go through a process called enrichment so that enough uranium-235 is present to use as a fuel for nuclear energy. Uranium-238 can be converted to a fissile substance called plutonium-239, in a nuclear reactor, and then used for fission. Another isotope of uranium that is fissile is uranium-233. It is not found naturally but can be produced from thorium.
| Properties | Uranium | Thorium |
|---|---|---|
| Chemical symbol | U | Th |
| Ore |
uraninite (UO2) coffinite U(SiO4)1-x(OH)4x brannerite UTi2O6 |
Thorium oxide (ThO2), monazite, carbonatite, thorite and thorianite |
| Name |
from the planet Uranus, itself named after Greek god of the sky Uranus | from Thor, the Norse god of thunder |
| Relative density | 19.1 g/cm3 | 11.7 g/cm3 |
| Hardness on Mohs scale | 6 | 3 |
| Malleability | High | High |
| Ductility | High | High |
| Melting point | 1132°C | 1750°C |
| Boiling point | 4131°C | 4788°C |
People will always be naturally exposed to small amounts of uranium and thorium through air, food and water, because these elements are common in soils and rocks. However, exposure to high levels of radiation from radioactive decay of high concentrations of uranium and thorium can lead to health problems in humans and other animals. Living cells that absorb too much radiation may be damaged or killed. While gamma radiation can easily go through human skin, alpha particles can only travel short distances and can't penetrate through skin.
Very high uranium intakes can cause kidney disease, kidney failure and death. Breathing in thorium dust increases the chance of developing lung disease and cancer of the lung or pancreas. In addition, thorium can be stored in bones, so it can cause bone cancer many years after exposure.
Uses
Uranium is used in nuclear reactors to generate electrical power. Electricity generation in nuclear reactors has many similarities to more conventional electricity generation in that the generated thermal energy is converted to mechanical energy that is used to drive turbo-generators (mostly by steam or superheated water). The breakdown of one atom of uranium produces more than one million times as much energy as is released by the combustion of a molecule of petrol.
Nuclear energy has been used as an energy source in some countries for six decades. Australia does not currently use nuclear energy but exports uranium to countries that do. Uranium is also used in the manufacture of radioisotopes for medical applications and in nuclear science research using neutron fluxes. Australia operates a nuclear research light-water reactor which produces medical radioisotopes at Lucas Heights in New South Wales.
In some countries, highly-enriched uranium is used by the military to fuel nuclear submarines, missiles and bombs. Depleted uranium is used in penetrating weapons and armour plating.
Thorium oxide (ThO2) has one of the highest melting points of all oxides and has been used in light bulb elements, arc-light lamps and welding electrodes as well as in heat resistant ceramics. However, non-radioactive substitutes have been developed for many applications of thorium. Yttrium compounds have replaced thorium compounds in lamps and lanthanides, zirconium, and yttrium can substitute for thorium in alloys used in the aerospace technologies.
History
1789 Martin Heinrich Klaproth discovered uranium in a sample of pitchblende (now called uraninite)
1828 Thorium was discovered by Morten Thrane Esmark on Lovoya Island in Norway. He sent the black mineral he found (later called thorite) to his father, a mineralogist named Jens Esmark, at the University of Oslo.
1829 Jöns Jacob Berzelius determined that the rock called thorite contained a new element, thorium
1896 Eugène-Melchior Péligot isolated the uranium metal and Henri Becquerel discovered its radioactive nature
1906 Mr Arthur J Smith pegged a claim at a uranium deposit in South Australia to mine carnotite (uranium ore). The deposit was used to produce radium, an element that was used to paint clock faces until the risks of its radioactivity were understood. The site eventually became Australia's first large uranium mine, Radium Hill, mined from 1954-1961
1934 A team of scientists that included Otto Hahn, Lise Meitner, Enrico Fermi and J. Robert Oppenheimer developed a way to use uranium as a fuel for producing nuclear energy
1940 All of the highest quality uranium ore extracted from a mine in the Congo was shipped to the USA because of fears that the ore might be seized by Germany
1945 The first nuclear weapon was developed and used in the USA
1946 The civilian Atomic Energy Commission was established in the USA to develop nuclear power
1949 The Soviet Union tested an atomic bomb and the subsequent nuclear arms race between the United States and the Soviet Union produced tens of thousands of nuclear weapons that used uranium metal
1950 Rum Jungle Uranium mining operations in Northern Territory began to support the British and US Governments nuclear weapons programs. This mine closed in 1971
1952 The United Kingdom conducted nuclear weapons tests in Australia between 1952 and 1957 at the Montebello Islands, Emu Field and Maralinga
1958 Mining began at the Mary Kathleen deposit in Queensland
1979 Nabarlek Mine opened in Northern Territory, after the Commonwealth Government announced that new uranium mines could be constructed to support nuclear power programs. The deposit was mined out in one dry season and is now rehabilitated
1981 Ranger Mine opened in Northern Territory
1988 An extensive underground mine was opened at Olympic Dam in South Australia, to mine copper and uranium
2001 The first in-situ leach mine, Beverley, opened in South Australia
Uranium has in the past been used to make yellow to green coloured glass that fluoresces green in ultraviolet light. It was also used for tinting in early photography.
Formation
Uranium and thorium are common elements in the Earth's crust. They can be found in low concentrations almost everywhere in rock, soil, rivers, and oceans. The Australian continent, through its geological heritage, is endowed with well-above-average concentrations of the uranium and thorium. Granites can have much higher levels of these elements than average crustal rocks, but still not high enough to be economical to mine.
The reason that granites have higher concentrations of uranium and thorium is because these elements are incompatible elements within magmas. This means that they are not stable in the structures of crystals and are easily replaced by other elements. So as magma cools, uranium and thorium are some of the last elements to be incorporated into crystals so the melt component of the magma becomes progressively enriched in uranium, thorium and other incompatible elements. In turn, these are the first elements to mobilise when rocks are heated or partially melted.
Uranium is highly soluble so it can be easily dissolved, transported and precipitated within ground waters by subtle changes in conditions. Uranium-rich minerals also dissolve easily, which is a further factor in the wide variety of geological conditions and places in which uranium mineralisation can occur.
In uranium deposits such as Beverley, Four Mile, and Mount Painter, the ore is hosted within permeable and porous sandstones or conglomerates. These deposits formed when groundwater containing dissolved uranium flowed into sedimentary rocks. The ground waters changed chemistry on contact with carbon-rich organic matter and, as a result, uranium oxide minerals were precipitated out into the porous rocks.
The uranium in the Yeelirrie deposit in Western Australia is hosted in a rock called calcrete within a salt-lake environment. This uranium was remobilised after weathering from granites that were formed 2.5 billion years earlier.
Thorium-rich minerals such as monazite are commonly found in igneous and metamorphic rocks. Monazite is a more resistant mineral so as rocks weather monazite grains remain intact. Eventually they are transported downslope by wind, water and gravity and can accumulate behind boulders, on the inside bends of stream channels or in the lower parts of a sediment deposit along with other heavy minerals.
Apart from heavy mineral sand deposits, thorium can be present in other geological settings such as alkaline igneous intrusions and complexes, including carbonatites, and in veins and dykes. In these deposits, thorium is usually associated with other commodities such as rare earths, zirconium, niobium, tantalum and other elements.
Resources
Australia has abundant uranium and thorium resources, with about 33% of the world's resources of uranium and 20% of the world's thorium.
Current uranium and thorium resources, production, consumption and trade information.
Uranium
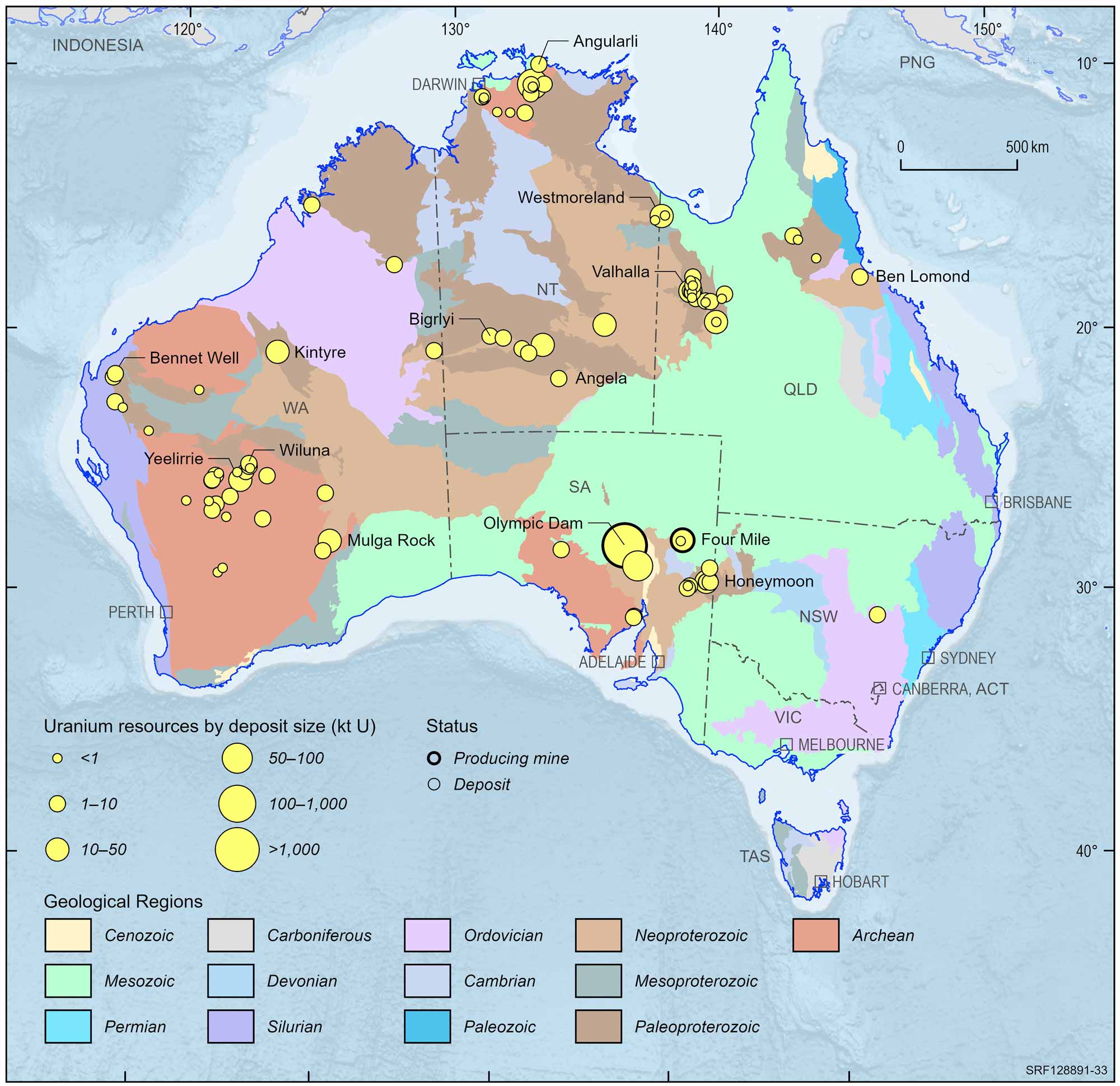
Uranium ore deposits can be found on all continents, with the largest deposits found in Australia, Kazakhstan and Canada. Uranium brings Australia annual export earnings of more than $1 billion. The giant Olympic Dam mine in South Australia is the world's largest uranium deposit. Uranium is also found at Beverley and Honeymoon in South Australia, in the Ranger and Jabiluka deposits of the Northern Territory, and at Yeelirrie in Western Australia. Queensland's three most prospective uranium deposits are inland from Townsville, in the Mount Isa region, and in the Gulf of Carpentaria region near the Northern Territory border. Uranium ore is found in veins and dykes at Nolans Bore deposit in the Northern Territory and in breccias in the Wolverine deposit in Western Australia.
Australian uranium deposits and operating mines, 2022.
Deposit size is based on total resources (EDR + Subeconomic Demonstrated Resources + Inferred).
For clarity, only major or significant deposits are labelled.
Some of the Australian uranium deposits are not currently accessible, including the Jabiluka deposit of Northern Territory where the traditional Aboriginal land owners have not granted approval to mine the deposit, and the Koongarra deposit, which was added to the Kakadu World Heritage Area by the World Heritage Committee in 2011. In South Australia, the Mount Gee deposit is within the Arkaroola Protection Area, established by the State Government in 2011, in which mineral exploration and mining are prohibited.
Uranium mined in Australia is mainly for export. Australia has no nuclear power stations, nuclear-powered vessels or nuclear weapons. Australian mining companies supply uranium to electricity companies in the USA, Japan, China, South Korea, Canada, United Kingdom, France, Germany, Spain, Sweden, Belgium and Finland. In addition, Australia has agreements with Russia, India and the United Arab Emirates to supply Australian uranium for use in their civil nuclear power programs.
Exports of Australian uranium are controlled by strict nuclear safeguards with other countries. Those safeguards specify that Australian uranium must be used exclusively for peaceful purposes in civilian nuclear fuel cycles. The material is also protected in accordance with internationally agreed standards for physical security. These agreements ensure that countries to which Australia sells uranium are committed to the safeguards and international nuclear security standards.
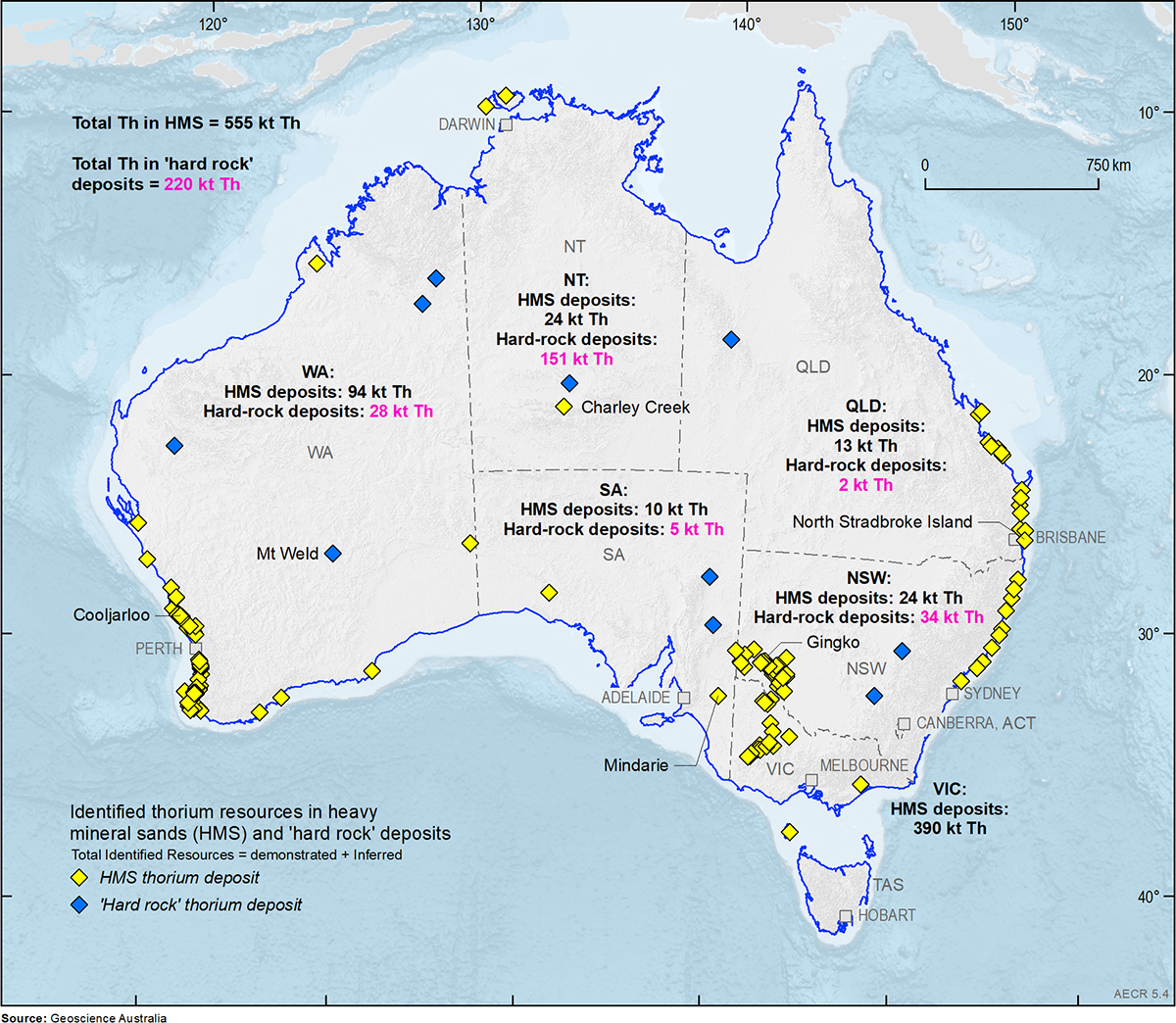
Thorium and monazite
Thorium is more evenly distributed than uranium, with significant deposits found in all states. Most known thorium resources in Australia are associated with the mineral monazite which is often found within heavy mineral sand and rare earth element deposits. Australia was once the world's largest producer of monazite and is thought to have the world's largest monazite resource.
Heavy mineral sands deposits are found in the Murray Basin, which includes parts of Victoria, New South Wales and South Australia, the Eucla Basin, in South Australia and Western Australia, and the Perth Basin, in Western Australia. Other mineral sand deposits occur in the north of Western Australia, Queensland, the Northern Territory and Tasmania. Most states in Australia host rare earth element deposits.
Between 1952 and 1995, Australia exported 265 kilotonnes (kt) of monazite mostly to France, but the monazite plant in France was closed because its operators were unable to obtain a permit for an associated toxic and radioactive waste disposal site. Current indications suggest that widespread use of thorium in nuclear reactors will not occur in the short to medium term, due to the challenges of technology development.
Further thorium resource and production information.
Mining
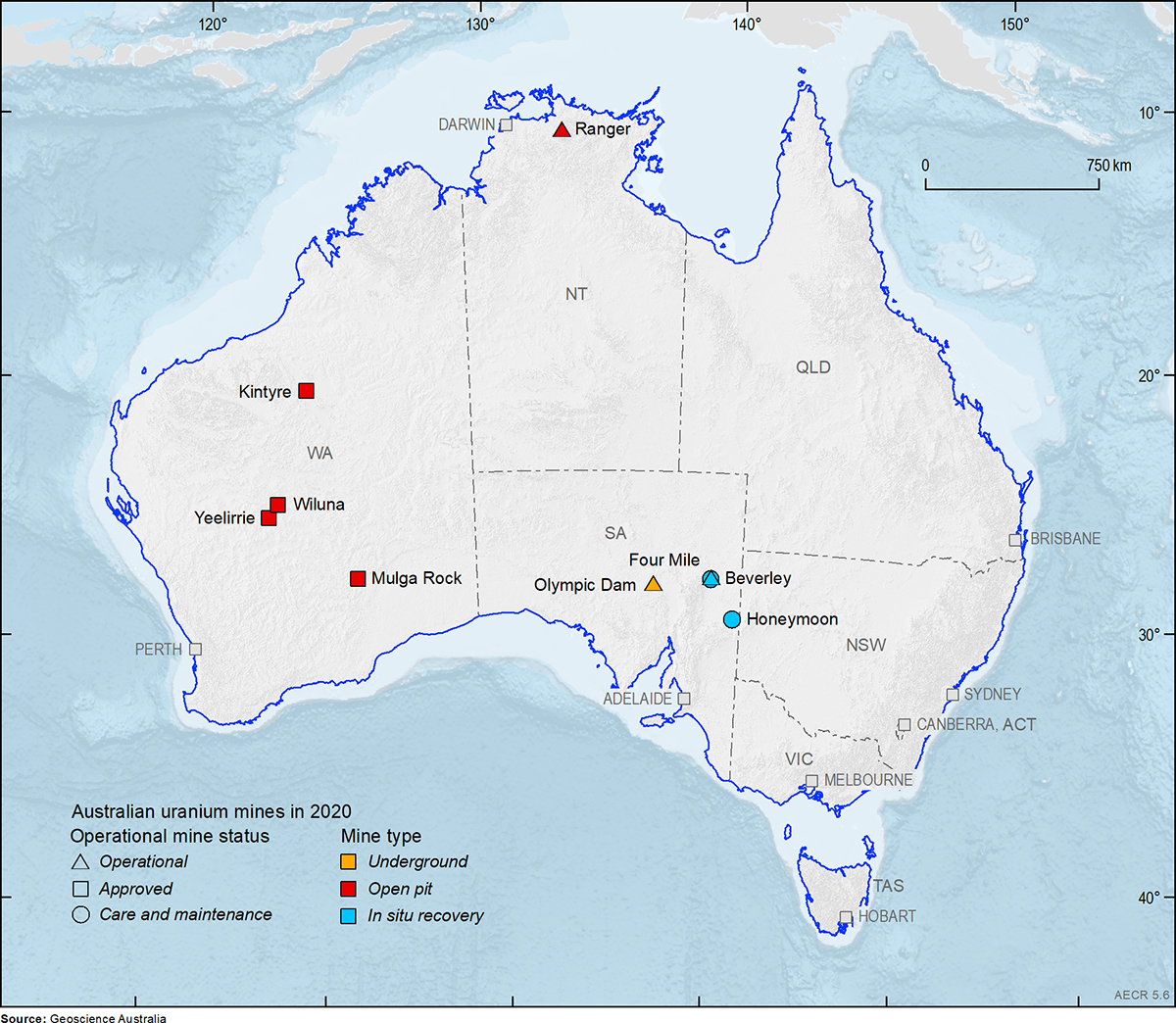
The ore at the Olympic Dam mine, South Australia is extracted by underground mining techniques whereas the Ranger Mine in Northern territory is an open pit mine. At the Beverley, Four Mile and Honeymoon deposits in-situ mining methods are used.
In-situ mining is also called in-situ leaching, in-situ recovery or solution mining. The technique involves dissolving minerals using either an acid or alkaline leaching solution which is pumped through the ore body. The solution dissolves the uranium and then these uranium-rich fluids are pumped to the surface where the metal is extracted. This means that conventional extractive mining techniques (open-cut or underground mining) are not required.
Significant uranium mining has not occurred in Queensland since 1982 due to a ban by the Queensland Government. However, exploration for uranium is still allowed.
There is no production of thorium in Australia but it is present in monazite being mined with other minerals in heavy mineral beach sand deposits. The heavy sands recovered are processed to separate these heavy minerals, and the light fraction is returned to the deposit. In current heavy mineral sand operations, the monazite fraction is returned to mine site and dispersed to reduce radiation as stipulated in mining conditions. Stream sediments, alluvial terraces, beach sediments, beach terraces, and shallow water sediments have all been mined for heavy minerals.
Processing
Uranium
Ore from in-situ uranium mines is already in a liquid form as it has been pumped out of the ground. However, ore from open-pit mines is first crushed and mixed with water and then added to large tanks of acid. Because uranium dissolves easily, in solution it becomes separated from other minerals and elements, which remain solid.
The uranium-rich solution is then purified and crystals of uranium oxide (U3O8) precipitate out. The resulting powder is called yellowcake, because early mining operations produced a bright yellow powder, but the yellowcake produced by most modern mills is brown or black. This is either sealed in drums for transport or processed further at the site. Uranium oxide is only mildly radioactive; it contains mostly (>99%) uranium-238. Australia exports uranium concentrate in this form.
The next step in concentrating the uranium is to separate impurities from the yellowcake. The powder is digested in nitric acid and water evaporated off. This produces high-purity uranium trioxide (UO3). Uranium trioxide can be smelted to form uranium dioxide (UO2) for use in fuel rods for heavy-water reactors.
Most nuclear power reactors require enriched uranium fuel where the proportion of uranium-235 has been increased from the natural levels to about 3-5%. The enrichment process involves converting the UO3 to uranium hexafluouride (UF6) by combining the compound with fluorine compounds. Enrichment plants use centrifuge systems, with thousands of spinning vertical tubes to separate uranium-235 from uranium-238. Highly enriched uranium, with uranium-235 levels higher than 20%, is suitable to power submarines and naval ships and levels above 90% are used for nuclear weapons.
Thorium and monazite
Thorium-bearing monazite extracted from mineral sands is usually mixed with a variety of other minerals, including silica, magnetite, ilmenite, zircon, and garnet. The first stage of concentrating the monazite is to wash out lighter minerals by placing the sand on shaking tables and passing the resulting monazite fraction through a series of electromagnetic separators.
To separate thorium from the other elements in monazite, the mineral is ground into a powder and mixed with hot concentrated sulphuric acid or sodium hydroxide solutions. The residue that is left contains 99% of the thorium and 5% of the other elements (mostly rare earths).
An alternative processing method involves converting the thorium in monazite, thorite, or other minerals to thorium dioxide (ThO2). This is then heated with calcium, sodium or magnesium. The compound produced is mixed with dilute nitric acid and then washed with water, alcohol, and ether. This produces a metal powder which can be compacted to form 99.7% pure thorium metal. Further processing can produce thorium metal that is 99.97% pure.