Magnesium
Page last updated:24 June 2022
Introduction
Magnesium is the eighth most abundant element in the Earth's crust. Elemental magnesium is a gray-white lightweight metal and occurs naturally only in combination with other elements. The metal can be produced artificially, but is highly reactive. When exposed to the atmosphere it is coated in a thin layer of oxide that partly inhibits reactivity. Magnesium is used in super-strong, lightweight materials and alloys.
Properties
Magnesium is the lightest of all metals, being about two-thirds lighter than aluminium. Magnesium is non-toxic, non-magnetic, has high-impact strength and is resistant to denting. Magnesium is too reactive to occur in nature as an element, but its compounds are common. Magnesium is used in super-strong, lightweight materials and alloys. For example, when infused with silicon carbide nanoparticles, it has extremely high specific strength.
The Properties of Magnesium
| Chemical symbol | Mg |
|---|---|
| Ore | Dolomite (a compound of magnesium and calcium carbonates, MgCO. CaCO3) and magnesite (magnesium carbonate, MgCO3) |
| Relative density | 1.74 g/cm3 |
| Hardness | 2 on Mohs scale |
| Malleability | High when heated |
| Ductility | High |
| Electrical conductivity | Good |
| Melting point | 650°C |
| Boiling point | 1107°C |
Uses
The largest single use for magnesium metal is in aluminium alloying, accounting for about 50% of the total magnesium metal consumption. The addition of magnesium to aluminium produces high-strength, corrosion-resistant alloys. About 20% is used in castings and wrought products including machinery, tools and other consumer products such as parts for cars. Magnesium is also useful in removing sulfur in the production of iron and steel, and the production of titanium in the Kroll process.
Because of its low weight and good mechanical and electrical properties, magnesium is widely used in the manufacturing of mobile phones, laptop and tablet computers, cameras, and other electronic components.
There are two main uses for magnesite (magnesium carbonate). The first is in the production of dead-burned magnesia for refractory brick use. These bricks are physically and chemically stable in high temperatures and are used to line furnaces in the steel industry, non-ferrous metal processing units and cement kilns. The second use involves processing to caustic calcined magnesia which is used principally as a food supplement in agribusiness and in fertilisers as well for fillers in paints, paper and plastics. Raw magnesite is used for surface coatings, landscaping, ceramics and as a fire retardant.
History
The name magnesium originates from the Greek word for a district in Thessaly called Magnesia.
In 1618, a farmer at Epsom in England attempted to give his cows water from a well. The cows refused to drink because of the water's bitter taste, but the farmer noticed that the water seemed to heal scratches and rashes. The substance became known as Epsom salts and its fame spread. It was eventually recognized as hydrated magnesium sulfate.
The metal magnesium was first produced by Sir Humphry Davy in England in 1808. He used electrolysis on a mixture of magnesia and mercuric oxide.
Historically, magnesium was one of the main aerospace construction metals and was used for German military aircraft as early as World War I and extensively for German aircraft in World War II.
Formation
Magnesite (MgCO3) is an ore for magnesium production. Magnesite occurs in two physical forms: cryptocrystalline or amorphous magnesite and macrocrystalline magnesite. It forms and occurs in five different ways:
- a replacement mineral in carbonate rocks;
- an alteration product in ultramafic rocks (igneous rocks composed mainly of one or more dark coloured ferromagnesian minerals);
- a vein-filling material;
- a sedimentary rock;
- as nodules formed in a lacustrine (lake) environment.
Replacement-type magnesite deposits involve magnesium-rich fluids entering limestone via openings to produce both magnesite and dolomite.
The alteration-type deposits are formed by the action of carbon dioxide-rich waters on magnesium-rich serpentinite a rock which has been formed from the alteration of magnesium and iron silicate minerals. The resulting magnesite may be very pure.
Sedimentary deposits usually occur as thin layers of variable magnesite quality. Lacustrine magnesite deposits consist of nodules of cryptocrystalline magnesite formed in a lake environment. Both vein filling and sedimentary magnesite occurrences are rarely mined on a large scale.
Magnesite, dolomite, carnallite, sea water and lake brines are all sources of magnesium metal. The largest magnesium resources are held in lake brines and sea water, however, most of the world’s magnesium production is sourced from magnesium-bearing minerals such as magnesite and dolomite.
When pure, magnesite contains 47.8% magnesium oxide and 52.2% carbon dioxide. Natural magnesite almost always contains some calcium carbonate as the mineral calcite and iron carbonate as the mineral siderite. Magnesium also occurs in dolomite, which has the formula CaMg(CO3)2
Resources
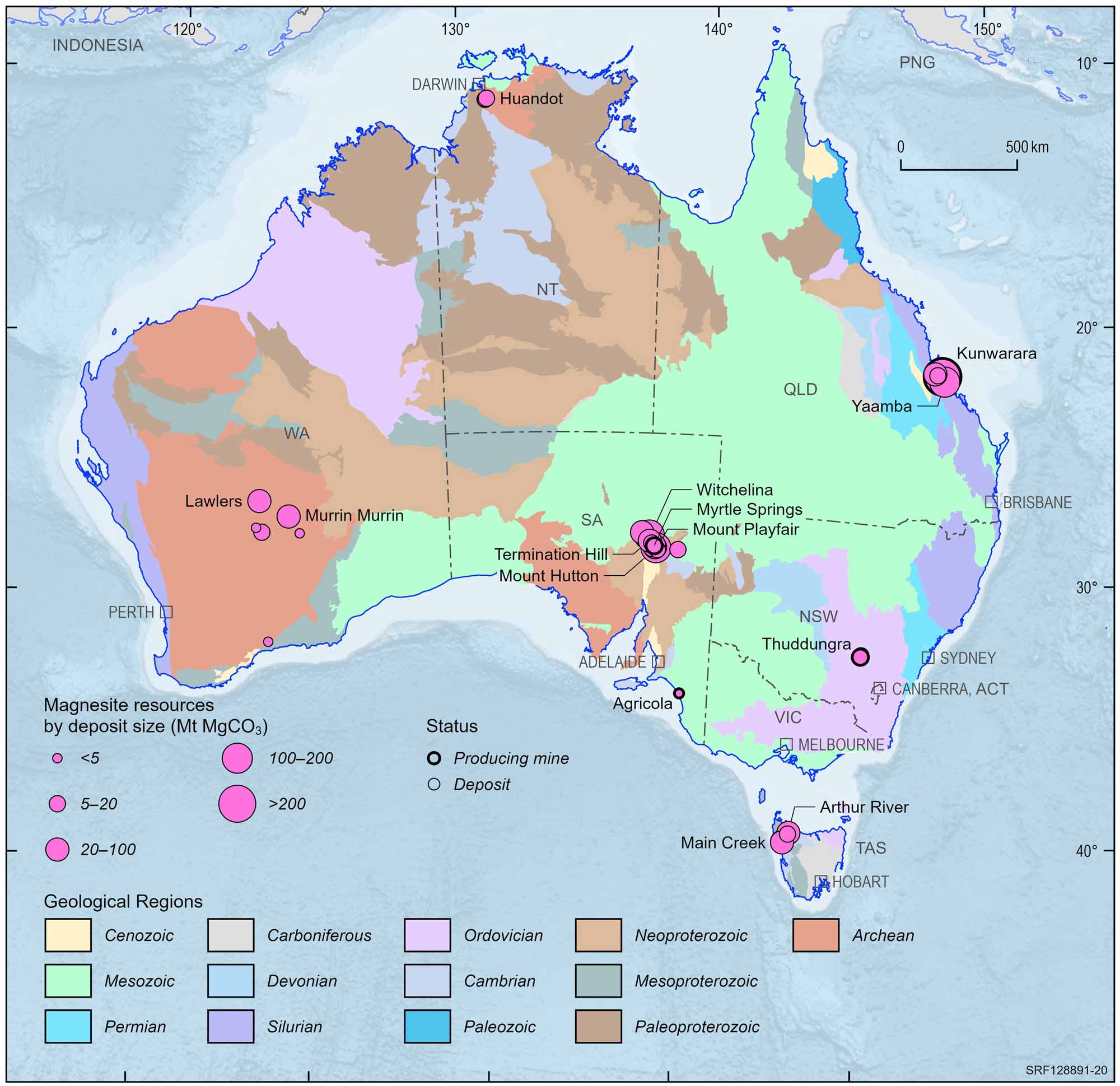
In the Kunwarara deposit, 60 km northwest of Rockhampton in Queensland, low iron nodules of cryptocrystalline magnesite cover an area of about 63 square km which is entirely overlain by black clay up to 12 metres thick. The deposit is thought to have formed by the deposition in lakes of magnesium bicarbonate derived from the alteration of serpentinite rock. Evaporation caused hydrated magnesium carbonate to precipitate. Deposition of mud over the magnesite resulted in further evaporation and the formation of hard nodules of dehydrated magnesite. Mining of this deposit commenced in 1989. Similar magnesite deposits occur at Yaamba and Triple Four, also in the Rockhampton area. Magnesite occurs near Gunnawarra southwest of Cairns, and in southern Queensland near Kilkivan and at Upper Widgee.
Australian magnesite deposits and operating mines, 2022.
Deposit size is based on total resources (EDR + Subeconomic Demonstrated Resources + Inferred).
For clarity, only major or significant deposits are labelled.
In New South Wales, magnesite at Thuddungra, northwest of Young, occurs as veins and nodules formed by the alteration of mafic rocks by magnesium-rich fluids. The magnesite ore contains 95 to 99% MgCO3 and varies in thickness from 2 to 10 metres. The Thuddungra deposit was first mined in 1935.
A former magnesite mine near Fifield about 30 km northwest of Condobolin consists of massive magnesite nodules occurring as pockets or veins in decomposed ultramafic rock. Other occurrences of magnesite in New South Wales are at Lake Cargellico, Cobar, Nyngan, between Attunga and Warialda.
In Tasmania, fine-grained, massive magnesite, formed by the replacement of limestone and dolomite, occurs at Arthur River and in the Lyons River area 50km south west of Burnie. The magnesite ore contains more than 40% magnesium oxide. Another deposit of magnesite also formed from the alteration of limestone is situated south of Arthur River at Main Creek.
In South Australia, beds of magnesite ranging from 5 centimetres to 9 metres in thickness occur at Witchelina, 80km north west of Leigh Creek, Copley, and Myrtle Springs. Magnesite also occurs at Balcanoona and near Robertstown.
In Western Australia, hard magnesite nodules in dark clayey material crop out 30km east of Ravensthorpe. Magnesite also occurs in the Kalgoorlie region.
Magnesite formed by the replacement of dolomite occurs at Huandot near Woodcutters in the Northern Territory. The deposit has an average width of 40 metres and an average overburden depth of 11 metres.
Mining
In Australia, all magnesite deposits are mined by open-cut methods. During mining the strip ratio, that is the quantity of waste material required to be removed in order to retrieve one tonne of magnesite ore, may be low. For example at the Kunwarara deposit in Queensland the strip ratio is low because the overburden averages only 4 metres thick. The majority of recorded magnesite production occurs in Queensland.
Processing
The processing of magnesite ore begins with crushing, screening and washing.
When magnesite is heated to between 700°—1000°C, carbon dioxide is driven off to produce caustic-calcined magnesia (caustic magnesia). Caustic magnesia is able to absorb liquids and to absorb heavy metals and ions from liquid streams making it useful in water treatment.
When calcined magnesia is heated to between 1530°—2300°C, the product produced is non-reactive and exhibits exceptional stability and strength at high temperatures. This product known as 'dead-burned' or 'sintered' magnesia is mainly used as a refractory material because of its inertness and high melting point.
When calcined or dead-burned magnesia is heated in excess of 2800°C in an electric arc furnace, electrofused magnesia is produced. It has higher strength, resistance to abrasion and greater chemical stability than dead-burned magnesia. It is used in the manufacture of premium grade refractory bricks used in the high wear hot spots of Basic Oxygen Furnaces, electric arc or similar furnaces where temperatures can approach 950°C.
Magnesia (magnesium oxide) is produced also from the processing of sea water and magnesium-rich brines but it is a very complex process using much larger amounts of energy than the process of heating of natural magnesite.
Magnesium metal can be produced by one of four processes:
- The electrolytic process uses magnesium chloride produced from either magnesite, seawater or brines rich in magnesium chloride. Magnesite is favoured as the source of magnesium because chlorine is recycled within the process rather than being disposed of as a waste or a by-product.
- The silicothermic process mixes calcined dolomite or magnesite with ferrosilicon (a combination of iron and silicon metal) to produce a magnesium vapour which is then condensed in cooling vessels to form magnesium metal.
- The Australian Magnesium Process developed in Australia involves dissolving pure magnesite ore in hydrochloric acid to produce magnesium chloride. The magnesium chloride is then purified, dehydrated to a dry feed and electrolysed in an Alcan cell. The molten magnesium is tapped from the cell and cast into ingots. The chlorine gas released is recycled and combined with hydrogen, from natural gas to produce hydrochloric acid for use in the process.
- The carbothermal reduction process is a relatively new method of producing magnesium metal and is a simple alternative to the more energy intensive silicothermic and electrolytic processes. In the carbothermal reduction process, carbon is used to reduce magnesium oxide, which produces a magnesium metal vapour and carbon monoxide gas by-products at temperatures above 1,600°C. The magnesium metal vapour is then cooled and condensed into a magnesium metal product.