Nickel
Page last updated:17 May 2018
Introduction
Nickel is not as well known as other metals but it plays an important, if invisible, role in modern life. When mixed with other metals nickel helps to create amazing alloys that are strong, won't rust, can withstand high and low temperatures and can be easily shaped into anything from thin wires to flat sheets. For example, nickel is one of the metals added to iron to make stainless steel - an extremely useful product. The Earth's magnetic field is due to the iron and nickel in its core.
Properties
Nickel is a hard silver-white metal with a high melting point and can withstand very low temperatures. Nickel is rarely found in the earth in its pure form; it mixes well with other metals to make many useful alloys. Nickel is malleable and ductile (can be beaten and drawn out into a wire) and is rust-resistant. Nickel is magnetic, although not as strongly as iron.
The Properties of Nickel
| Chemical Symbol | Ni - Nickel's name comes from the Saxon term 'Kupfernickel' or Devils' Copper, as the 15th century miners thought the ore looked red-brown like copper and that it was too difficult to mine and was poisoning them (actually it was arsenic doing this). |
|---|---|
| Mineral | Most often in combination with sulfur and iron in pentlandite, with sulfur in millerite, with arsenic in the mineral nickeline, and with arsenic and sulfur in galena. |
| Relative density | 8.9 g/cm3 |
| Hardness | 4 on Mohs scale |
| Malleability | High |
| Ductility | High |
| Melting point | 1455°C |
| Boiling point | 2730°C |
Uses
Nickel-cadmium rechargeable batteries are used to power mobile phones, radios, clocks and calculators.
| Use | Description |
|---|---|
| Alloys | More than 80% of nickel is used to make alloys, as nickel adds toughness, strength, rust resistance and various other electrical, magnetic and heat resistant properties. At least 3000 nickel alloys have been created, including stainless steel. These alloys are used for many purposes such as in construction, the chemical industry, cars (crank-shafts and axles), household products (kitchen sinks, cooking utensils, washing machines etc), propeller shafts, scientific and surgical equipment, pipelines and aircraft engines. |
| Batteries | Nickel-cadmium rechargeable batteries are used to power mobile phones, radios, clocks and calculators. |
| Coins | Nickel was first used for coinage in Belgium in 1860, and has been widely used since then. Australian $1 and $2 coins contain 2% nickel (with 92% copper and 6% aluminium), and our 5c, 10c, 20c and 50c coins contain 25% nickel (with 75% copper). The Australian 20 cent coin is more silver-white in colour than a $1 coin due to the relative amounts of nickel. |
| Other |
|
History
The ancient Chinese used nickel alloys (calling them 'paktong'); however, it was 1751 before nickel was first isolated by a Swedish chemist, Alex Cronstedt.
In the 19th century, nickel was popular for making items such as cutlery. In 1889, James Riley gave an historic speech to the Iron and Steel Institute of Great Britain, declaring that tests had shown that a steel containing nickel gave the alloy almost unbelievable strength. From then on, nickel alloy steels became vital materials for a whole range of uses.
There was an especially large demand for nickel during the two World Wars for making armour plate for vehicles.
Formation
Most mined nickel derives from two types of ore deposits that form in very different geological environments: magmatic sulfide deposits, where the principal ore mineral is pentlandite [(Ni,Fe)9S8], and laterites, where the principal ore minerals are nickeliferous limonite [(Fe,Ni)O(OH)] and garnierite (a hydrous nickel silicate). Magmatic nickel sulfide deposits form when magmas derived from the Earth’s mantle ascend into the crust, in some cases reaching the Earth’s surface, and crystallise into iron-magnesium-nickel-rich mafic and ultramafic rocks containing concentrations of Ni-rich sulfide minerals. In some deposits the Ni is associated with concentrations of platinum-group elements (PGEs) and copper, which increase the value of the nickel ore deposits. For example, the Noril’sk Ni-Cu-PGE deposit in Russia is estimated to have a value of approximately $1 trillion, representing one of the most valuable deposits in the world. Most of Australia’s nickel sulfide resources occur in Western Australia within ultramafic lava flows of Archean age. Other countries with major resources of nickel sulfide deposits include South Africa, Canada and Russia.
Laterite-hosted nickel deposits form by the weathering of ultramafic rocks and are a near-surface phenomenon related to tropical climates. Although Australia holds large resources of lateritic nickel, very large deposits of this type also occur in Indonesia, New Caledonia, Philippines, Cuba and Brazil.
Resources
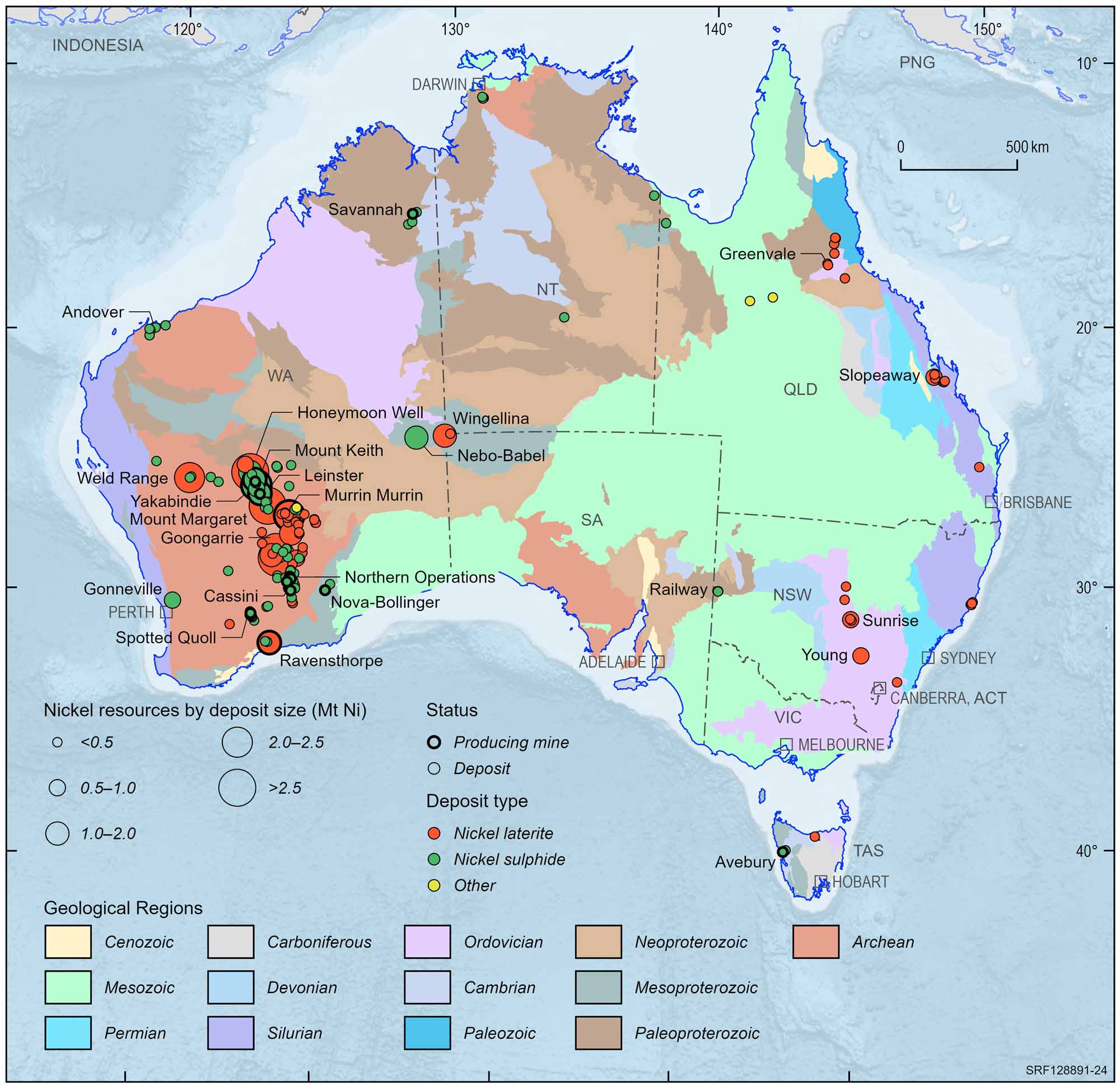
The sampling of some curious green-stained iron-rich rocks from the shores of Lake Lefroy in the Eastern Goldfields of Western Australia in 1947 represents an important event in the evolution of the nickel industry of Australia. Follow-up exploration led to the discovery in 1966 of the first major nickel sulfide deposit in Australia, heralding the initiation of Australia's nickel mining industry, an industry which has defined five world-class deposits (> 1 Mt contained nickel).
Western Australia has the largest nickel resources with 96% of total Australian resources, contained within nickel sulfide and lateritic nickel deposits. Queensland is the second largest with 4.5% (lateritic nickel), followed by Tasmania with 0.2%. Currently, all nickel is accessible for mining.
Australian nickel deposits and operating mines, 2022.
Deposit size is based on total resources (EDR + Subeconomic Demonstrated Resources + Inferred).
For clarity, only major or significant deposits are labelled.
Mining
The size, grade, morphology and depth of the ore deposits determine whether they are mined by underground or open-pit methods. Lateritic nickel deposits are generally mined from open pits by strip mining, due to the shallow depth of most deposits and their clayey nature. The overburden is removed and generally deposited in the pit created from earlier mining.
Underground extraction uses a variety of standard mining methods, and may be combined with open-pit mining simultaneously at the same deposit in order to access both shallow and deep parts of the deposit.
Processing
After mining, nickel ores are further processed to upgrade their nickel contents from 1-4% typical of the ores to concentrates with grades in the range 10-20%. Concentration involves crushing the ore and separating nickel-bearing from other minerals using various physical and chemical processing methods. The concentrates are then smelted (in a furnace) to produce a sulfide ‘matte’ with up to 70% Ni. The final stage of processing is refining, involving pyrometallurical (heating) and hydrometallurgical (chemical) processes, which are utilised differently for nickel sulfide and lateritic nickel ores.