Zinc
Page last updated:17 May 2018
Introduction
Mostpeople have put zinc cream on their noses at one time or another to protect their skin from sunburn. But did you know that zinc is called 'The Great Protector' for another reason; zinc protects iron and steel from corrosion, very important when you think that almost all our buildings, railways, lighting pylons, cars, and bridges (to name but a few things) contain steel. Without zinc, we would live in a very rusty world! We would also have to do without lots of products we take for granted such as brass.Australia uses more zinc-coated steel (like Colorbond or Zincalume) per person than any other country.
Properties
Pure zinc is a bluish-white, shiny metal which is resistant to corrosion.
Zinc has never been found naturally in its pure form. It can be alloyed (mixed) with a number of other metals. Zinc is brittle at ordinary temperatures but is malleable and ductile (can be beaten and drawn into a wire) when heated to 1000°C. Zinc has a relatively low melting point and is a good electrical conductor.About 30% of zinc used in the Western World comes from recycled materials.
The Properties of Zinc
| Chemical Symbol | Zn |
|---|---|
| Ore | Sphalerite |
| Relative density | 7.14 g/cm3 |
| Hardness | 2.5 on Mohs scale |
| Malleability | Brittle, but high when heated |
| Ductility | Low ductility, but high when heated |
| Melting point | 420°C |
| Boiling point | 907°C |
Uses
The construction, transport and appliance manufacturing industries use large amounts of zinc, mainly as anti-corrosion coatings (galvanising) on sheet steel, steel beams, vehicle panels, chain-link fencing, guard rails and light posts. World-wide, galvanising accounts for about half of the world's total consumption of zinc. The widespread use of zinc as a protective coating is mainly because of its resistance to weathering as a consequence of an electrochemical reaction known as galvanic action. Zinc is more reactive than iron or steel and consequently attracts almost all local oxidation. A protective surface layer of oxide and carbonate forms as the zinc corrodes. Most of the rest of zinc consumption is as brass and other alloys, and the balance in other uses such as pigments, salts, oxide additives to rubber and agricultural chemicals.
| Use | Description |
|---|---|
Galvanising | About two-thirds of all the zinc used in Australia today is to protect steel from rusting, by coating it using a process called galvanising (named after the Italian chemist, Luigi Galvani, who invented the process). The steel is dipped in molten zinc, often also with aluminium. Products include steel beams, roofs, poles, wires, nails, household appliances and car bodies. |
Diecast objects | Zinc mixed with small amounts of aluminium produces a very strong alloy. Its low melting point enables it to be diecast (cast into different shapes in steel moulds) to make all sorts of items (some requiring fine detail) from carburettors to door handles, staples to zips, even MatchboxÔ cars. |
Brass | Brass (70% copper, 30% zinc) is particularly rust-resistant and so is used to make the hulls of sailing boats and other marine hardware. Many musical instruments are made from brass. Brass is also used to make decorative pieces, from light fittings to taps, and instruments for astronomy, surveying, navigation and other scientific purposes. |
Batteries | When alloyed with other metals, zinc becomes a good electrical conductor. Zinc is also used in the very common zinc-carbon battery, and zinc-bromide and zinc-nickel power cells are amongst the newest types of batteries. |
Health | Humans and other animals need to ingest zinc for proper growth and healing of wounds to occur. Fruits, nuts, meat, oysters and other shellfish are good sources of zinc. |
Zinc oxide | Zinc oxide is a unique and very useful material, used in the manufacture of rubber tyres, skin products (such as zinc cream, anti-dandruff shampoos, antiseptic ointments and calamine lotion for healing skin disorders), paints, floor coverings, plastics (to help prevent them cracking) and ceramic glazes. |
Zinc sulphate | In luminous dials on watches, TV screens and fluorescent lights. |
Other zinc compounds | As a dissolving agent, to help prevent plastics from cracking, in surgical dressings, glues, and to preserve and fire-proof timber. Zinc dust is very flammable when dry so it is used in fireworks. |
History
Zinc (Zn) was used in Rome and China more than 2000 years ago as a component of brass which is a zinc-copper alloy. The Romans and Chinese smelted zinc ores such as calamine (zinc carbonate) with copper to produce brass, used for coins, containers, armour and jewellery. They did not actually realise that zinc was a metal. The Romans also used calamine for healing wounds.Pure zinc was probably first produced in India and China in the 13th or 14th century.Commercial production of zinc did not start in Europe until the middle of the 18th century and until 1860 in the United States.
Formation
The main zinc mineral is sphalerite ((Zn,Fe)S), which contains up to 67% zinc by mass. Smithsonite (ZnCO3, 52%), willemite (Zn2SiO4, 59%) and hemimorphite (Zn4Si2O7(OH)2 H2O, 54%) may occur in the near-surface weathered or oxidised zone of an orebody. In some cases, these secondary minerals are also zinc ores.
Deposits containing zinc form from hot, aqueous (or hydrothermal) fluids generated within the earth. These fluids may flow along sub-surface fractures where sphalerite and other minerals may precipitate to make vein deposits. Where limestones occur, the fluids may flow through cavities to form rich but patchy deposits. Some fluids may reach the ocean floor in areas of underwater volcanic activity to form volcanogenic deposits. Examples of this last type of deposit are forming today under the oceans off Papua New Guinea, Canada and elsewhere in the world. Other fluids may escape to the surface through fractures into shallow seas and under suitable conditions, lead-zinc-silver deposits may form.
For thousands of millions of years, deposits have been forming in these manners. These deposits may eventually be exposed at the surface following weathering and erosion. Some are completely eroded away and may be recycled by natural processes into the earth. Partially eroded deposits were relatively easy to discover; examples include deposits found at Broken Hill in New South Wales in the late 1800s and at Mt Isa in Queensland last century. These deposits formed the basis of Australia's zinc mining industry.
Resources
In deposits mined today, zinc ore (rock containing economic content of zinc and/or other materials) commonly also contains lead, silver and, in some cases, copper, and the zinc is extracted as a co-product along withthese other metals.The discovery of a large zinc-lead-silver orebody at Broken Hill in 1883 (a mine is still in operation today) was a significant event in Australia's history as it greatly boosted the economy. (see Google Arts and Culture -Broken Hill: one of the world's largest lead-zinc-silver deposits). Previously Australia had relied only on wool and wheat for the nation's wealth.
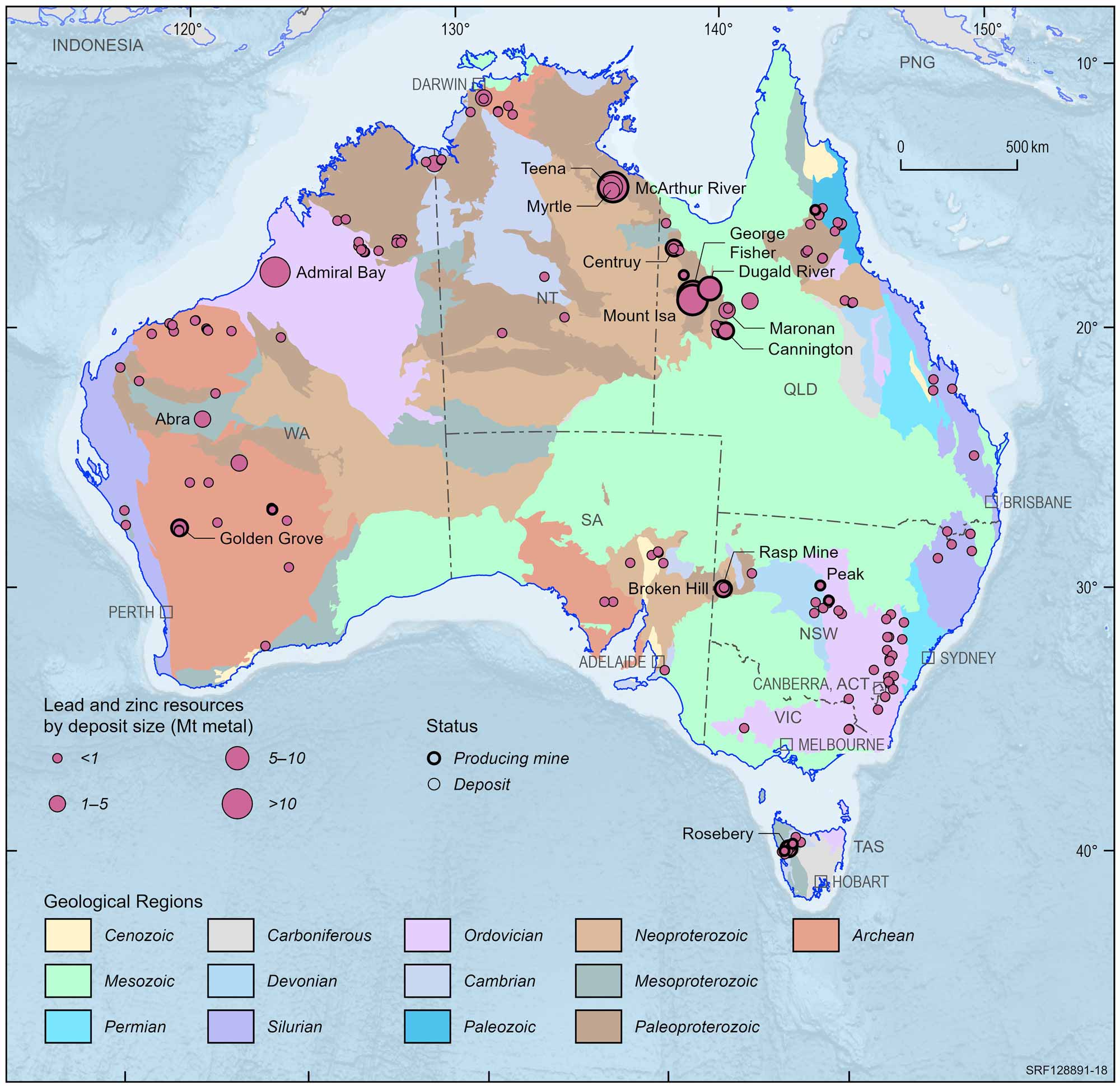
Australian lead and zinc deposits and operating mines, 2022.
Deposit size is based on total resources (EDR + Subeconomic Demonstrated Resources + Inferred).
For clarity, only major or significant deposits are labelled.
The rich lodes at Mt Isa were not discovered until 1923 and were developed despite the remote location and harsh environment. The nearby, rich Hilton deposit was discovered in the late 1940s but not developed until the mid-1980s. Production at Mt Isa continues and it has been the second biggest zinc producer in Australia. In the Mt Isa region, there are large mines at the George Fisher, Cannington and Century deposits, while the Dugald River and Lady Loretta deposits are yet to be developed. In the Northern Territory, the large McArthur River deposit is a major producer.
Zinc ore is produced also at Rosebery in Tasmania; Elura in New South Wales; and Scuddles and Gossan Hill in Western Australia. High-grade zinc silicate ore has been mined intermittently from the small Beltana deposit in South Australia.
Exposed deposits are becoming harder to find in Australia and exploration companies are currently looking beneath the surface for the deposits of the future which is a more costly and difficult way to find orebodies. However, there have been a series of successes since the late 1970s including the Scuddles mine (140 m deep) and Admiral Bay deposit (1.5 km deep) in Western Australia, the Cannington deposit (10 m deep) in north Queensland, the mined-out Hellyer deposit (90 m deep) in Tasmania, the Wilga deposit (50 m deep) in Victoria and the Teena deposit (500 m deep) in the Northern Territory.
Australia today has more than 20% of the world's known zinc-lead resources and is the largest producer and exporter of these metals to the rest of the world. Our main zinc mines are at McArthur River in the Northern Territory and Hilton-George Fisher in Queensland. Dugald River is also being developed in north-west Queensland on one of the world's highest grade known zinc deposits. Australia ranks third behind China and Canada in world mine production and second to Canada in exports of zinc. Australia exports zinc as refined metal to a broad range of destinations in the Asia Pacific area extending from India to the USA, but mainly to Indonesia, Hong Kong, Chinese Taipei and Malaysia. The major customers for Australian zinc in ores and concentrates are Japan and South Korea, and to a lesser extent Belgium, Germany and the United Kingdom.
Mining
Australia is at the forefront of technological development in zinc mining and processing. Almost all of Australia's zinc mines are underground operations and are highly mechanised. Ore is drilled and blasted in large volumes, transferred to underground rock crushers by large loaders and trucks before being hoisted to the surface in skips or driven directly to the surface by truck via a spiral access tunnel called a decline.
At the surface, the ore is subjected to additional crushing and fine grinding. The flotation process is then used to separate the zinc and other valuable sulphide minerals from the waste rock particles or tailings to form a concentrate.
Processing
In the early days of Broken Hill virtually none of the zinc could be extracted economically and zinc minerals were sent to the waste dumps. Gravity and magnetic separation methods were unsuccessful but in 1901 a new metallurgical process, called flotation, was devised at Broken Hill which achieved recoveries of the zinc minerals from ore upwards of 60%. After considerable experimentation, a selective flotation method which worked on a commercial scale was perfected in 1912. Improved versions of this flotation process, such as the Australian-developed Jameson flotation cell are used world-wide today.
The Jameson flotation cell is installed in many mines around Australia. Ground ore, water and special chemicals are mixed and constantly agitated in banks of flotation cells. Air is blown through the mixture in each cell, and the fine zinc sulphide particles stick to the bubbles which rise to form a froth on the surface of the flotation cell. The tailings sink and are removed from the bottom of the cell. The froth is skimmed off and the resulting zinc sulphide concentrate is dried. This process upgrades the ore, which may contain only 6% zinc, to a concentrate assaying more than 50% zinc. Up to 90% of the zinc in the ore can be recovered.
Electrolysis and smelting are the two processes used to produce zinc metal. In Australia, the electrolytic process is currently used at the Risdon zinc refinery in Tasmania and the Sunmetals refinery in Townsville. At both plants zinc concentrate from various Australian mines is roasted to remove most of the sulphur as sulphur dioxide and make impure zinc oxide. The roasted concentrate is then leached with sulphuric acid to form zinc sulphate solution. The zinc sulphate solution is purified by adding a small amount of zinc powder to precipitate and remove traces of copper, cadmium, cobalt and nickel. The solution is piped to electrolytic cells where the zinc is electrochemically deposited on aluminium cathodes (electrodes). The zinc is removed from the cathodes, melted in a furnace and cast into slabs.
Beginning with the development of the Jameson flotation cell early last century, Australia has been at the forefront of technology for the mining, milling and extraction of zinc and other related metals. Recent Australian advances include development of the Isasmelt and Isamill technologies, which were developed to address problems associated with processing Zn-Pb ores from the North Australian Zinc Belt. The Isasmelt technology was developed in the 1970s to the 1990s to better meet more stringent environmental controls for lead smelting. This technology has since been transferred to the smelting of other metals, notably copper, and is used throughout the world. The Isamill process, which uses ceramic balls, sand or slag rather than steel balls to grind ore, was developed in the 1990s to allow the very fine grinding of ore required to liberate sulphide minerals from the very-fine-grained McArthur River (HYC) ores. Since 2003 the Isamill technology has been used around the world to produce a large range of commodities, including platinum, copper, gold, molybdenum and magnetite.
Primary refined zinc is or has been produced at four plants in Australia - Risdon in Tasmania, Cockle Creek in New South Wales, Port Pirrie in South Australia, and Townsville in Queensland. Small production of secondary refined zinc occurs at Port Kembla in New South Wales. Zinc oxide and zinc dust is produced from primary and scrap zinc at West Footscray in Melbourne, Victoria and in minor amounts in Brisbane, Queensland.
At both the Risdon and Sunmetal smelters, zinc concentrates are initially roasted to remove sulfur an produce a zinc calcine. This calcine is leached using acid, and the resulting leachate is purified by adding zinc dust. Zinc is extracted from this solution electrolytically to produce cathode zinc, which melted in furnaces to produce ingots. Risdon is Australia's largest zinc refinery and also one of the largest in the world.Less than half of Australia's zinc concentrates are processed domestically.
Historically, smelting was used at the Cockle Creek smelter near Newcastle in New South Wales to produce zinc and lead metal simultaneously in a blast furnace. Zinc and lead concentrates from various mines were blended and sintered (partly melted) to combine the fine particles into lumps and remove some sulphur as sulphur dioxide. The sintered product was mixed with coke and smelted in a blast furnace to produce zinc vapour (gas), which was condensed by cooling with a spray of molten lead to form impure molten zinc metal (98.3% zinc). To remove the small amount of lead and cadmium impurities the liquid zinc was twice boiled to zinc vapour and recondensed to produce high purity zinc metal (up to 99.95%).
At Port Pirie, zinc was recovered from the lead smelter slag or molten waste, which contains about 17% zinc, and also from residues from the Risdon zinc refinery. The molten slag was heated further to drive off zinc and some lead vapour, which was oxidised to form a zinc oxide fume and filtered out as dust in a bag filter. This dust was ground and put through an electrolytic refining plant to produce high purity zinc.