Copper
Page last updated:7 September 2023
Introduction
Every time you switch on a light, use an appliance in your home, or turn on a tap, it is copper that is delivering the electricity or water to you. Copper is therefore a very important metal to humans and combines more useful properties than probably any other metal.
An average family home contains more than 90 kilograms of copper: 40 kg of electrical wire, 30 kg of plumbing, 15 kg of builder’s hardware, 9 kg inside electrical appliances and 5 kg of brass goods. A Boeing 747-200 jet plane contains about 1.8 tonnes of copper. The Statue of Liberty in New York contains more than 27 tonnes of copper.
Properties
Copper is the only naturally occurring metal other than gold that has a distinctive colour. Like gold and silver, copper is an excellent conductor of heat and electricity. It is also very malleable and ductile. Copper is also resistant to corrosion (it does not rust very easily). Copper is soft but tough. It is easily mixed with other metals to form alloys such as bronze and brass. Bronze is an alloy of tin and copper and brass is an alloy of zinc and copper. Copper and brass are easily recycled¿perhaps 70% of the copper now in use has been recycled at least once.
The Properties of Copper
| Chemical symbol | Cu, from the Latin word 'cuprum', which means 'ore of Cyprus'. |
|---|---|
| Ore | Most commonly found as chalcopyrite, CuFeS2 |
| Relative density | 8.96 g/cm3 |
| Hardness | 3 on Mohs scale |
| Malleability | High |
| Ductility | High |
| Melting point | 1084°C |
| Boiling point | 2562°C |
Uses
Today copper, because it is such a good conductor of electricity, is used in electrical generators and motors for electrical wiring and in electronic goods, such as radios and TVs. Copper also conducts heat well, so it is used in motor vehicle radiators, air-conditioners and home heating systems.
As copper does not corrode easily it is also used for water pipes. Its malleability means that copper pipes can be bent to go round corners easily, without breaking.
Copper sulphate is used as a fungicide to stop plant roots from blocking drains and sewerage systems. The blue-green colour of treated timber is the result of copper naphthanate and copper-chrome-arsenate which have been introduced under pressure to help protect the wood from borers.
Copper is also used for making coins and scientific instruments as well as in decorative applications.
There is about 15 grams of copper in a mobile phone and recently copper has been replacing aluminium in computer chips.
| Use | Description |
|---|---|
| Electricity and communication | As copper is ductile and a great conductor, its main use is in electric generators, household/car electrical wiring, and the wires in appliances, computers, lights, motors, telephone cables, radios and TVs. |
| Coins | The alloy 'cupronickel', a mixture of 75% copper and 25% nickel, is used for making 'silver' coins such as the Australian 5, 10, 20 and 50 cent pieces. Australia's $1 and $2 coins are 92% copper, mixed with aluminium and nickel. |
| Pipes | As copper does not rust easily and can be easily joined, it is useful for making water pipes (and hydraulic systems). The use of copper in water pipes dates back to the ancient Egyptians and the Romans. |
| Heat conducting | Copper's ability to conduct heat means it is used for car radiators, air conditioners, home heating systems and boilers to produce steam. It is also ideal for the base of cooking pots. |
| Fungicides and insecticides | Copper sulphate is used to kill algal blooms in water reservoirs, to protect timber, to stop plant roots from blocking rains and sewerage systems and to kill insects. |
| Fertilisers | Copper production was boosted in the 1950s and 1960s by the need for copper-based fertilisers to aid crop growth in previously unproductive land. |
| Bronze | Bronze (90% copper, 10% tin) is used for statues and bearings in car engines and heavy machinery. The earliest bronzes were natural alloys derived from mineral deposits which also contained tin. |
| Brass | Brass (70% copper, 30% zinc) is particularly rust-resistant and so is used to make the hulls of sailing boats and other marine hardware. Many musical instruments are made from brass. It is also used for decorative pieces, from light fittings to taps and instruments for astronomy, surveying, navigation and other scientific purposes. |
History
Copper was the first metal used by people. It was discovered by Neolithic Man about 9000 years ago and used in place of stone, as it was far easier to shape. Early coppersmiths in Iran found that heating copper softened it and hammering copper made it harder. In this way, they could shape copper into various useful items such as containers and utensils¿a big leap forward for the human race. Its beautiful colour also made copper attractive for use in jewellery and ornaments.
There is evidence that copper was used from early times, a piece of copper tubing used 5000 years ago was unearthed by archaeologists from the Pyramid of Cheops in Egypt. Around 4000 BC, bronze (an even harder alloy) was discovered by mixing copper with a small amount of tin. It was used to make weapons, armour, tools and decorations tools¿thus began the Copper-Bronze Age. Although the manufacture of bronze tools largely fell into disuse with the onset of the Iron Age about 1000 BC, copper continued to be used for its other properties. As one of only two coloured metals, its beauty makes it highly desirable for making ornaments and its resistance to corrosion makes it suitable for use in, or near the sea.
The ability to beat copper into sheets and its resistance to rusting made it a popular roofing material on important buildings.
The growth of the copper industry has been closely linked with the increasing use of electricity. Electrical applications continue to be the metal's principal use, which can be attributed to two physical properties. It is an excellent electrical (and heat) conductor and is ductile enough to be drawn into wire and beaten into sheets without fracturing. Copper is used widely in plumbing components and is a major component of alloys, many of which are harder, stronger and tougher than their individual constituent elements. In 1837 Charles Wheatstone and William Cooke patented the first electric telegraph, using copper wire. In 1876 Alexander Graham Bell was the first to use copper telephone wire. In 1878 Thomas Edison invented the first electric light, relying on copper to carry the current to it. Within a few years, the mass use of these two inventions caused an incredible increase in the mining and production of copper.
Formation
Because copper reacts readily with other substances, it can be formed in a variety of ways in the Earth's crust. It is often found in deposits with other metals such as lead, zinc, gold and silver.
By far the largest amounts of copper are found in the crust in bodies known as porphyry copper deposits. These deposits were once large masses of molten rock that cooled and solidified in the Earth's crust. As they cooled, some large crystals grew, which were then surrounded by smaller crystals as cooling became more rapid - geologists call these rocks porphyries. At first, the copper was spread throughout the large mass of molten rock in low concentrations. As the magma cooled and crystals began to form, the amount of melt became smaller. The copper remained in the melt, becoming more and more concentrated. When the rock was almost completely solid, it contracted and cracked and the remaining copper-rich fluid was squeezed into the cracks, where it too finally solidified. Over many millions of years the rocks covering these deposits eroded away and the deposits eventually appeared at the surface. Examples of porphyry deposits include Cadia Hill (NSW) and Cerro Colorado (Panama).
A mixture of copper, iron and sulfur is called chalcopyrite (CuFeS2) or 'fool's gold', and tricked many an old-time prospector! Chalcopyrite in Australia is found in rocks that are more than 250 million years old. Bornite (Cu5FeS4), covellite (CuS) and chalcocite (Cu2S) are important sources of copper in the world and many ore bodies also contain some malachite (CuCO3.Cu(OH)2), azurite (Cu3(CO3)2.Cu(OH)2), cuprite (Cu2O), tenorite (CuO) and native copper. The sulfides, which yield most of the copper produced throughout the world, generally occupy the deeper parts of lodes which have not been exposed to weathering. Near the surface they are altered by oxidation and other chemical actions to produce oxides and carbonates. These secondary copper minerals may form rich ore in the upper parts of many deposits, and owing to their characteristic green or blue colour, even small amounts are easily seen in the rocks in which they occur. Copper bearing minerals are commonly found in association with minerals which may contain gold, lead, zinc and silver.
Resources
In Australia, the search for copper began soon after European settlement. The first major discovery of copper in Australia was at Kapunda in South Australia in 1842 when Francis Dutton found copper ore whilst searching for lost sheep. By the 1860s, South Australia was known as the 'Copper Kingdom' because it had some of the largest copper mines in the world.
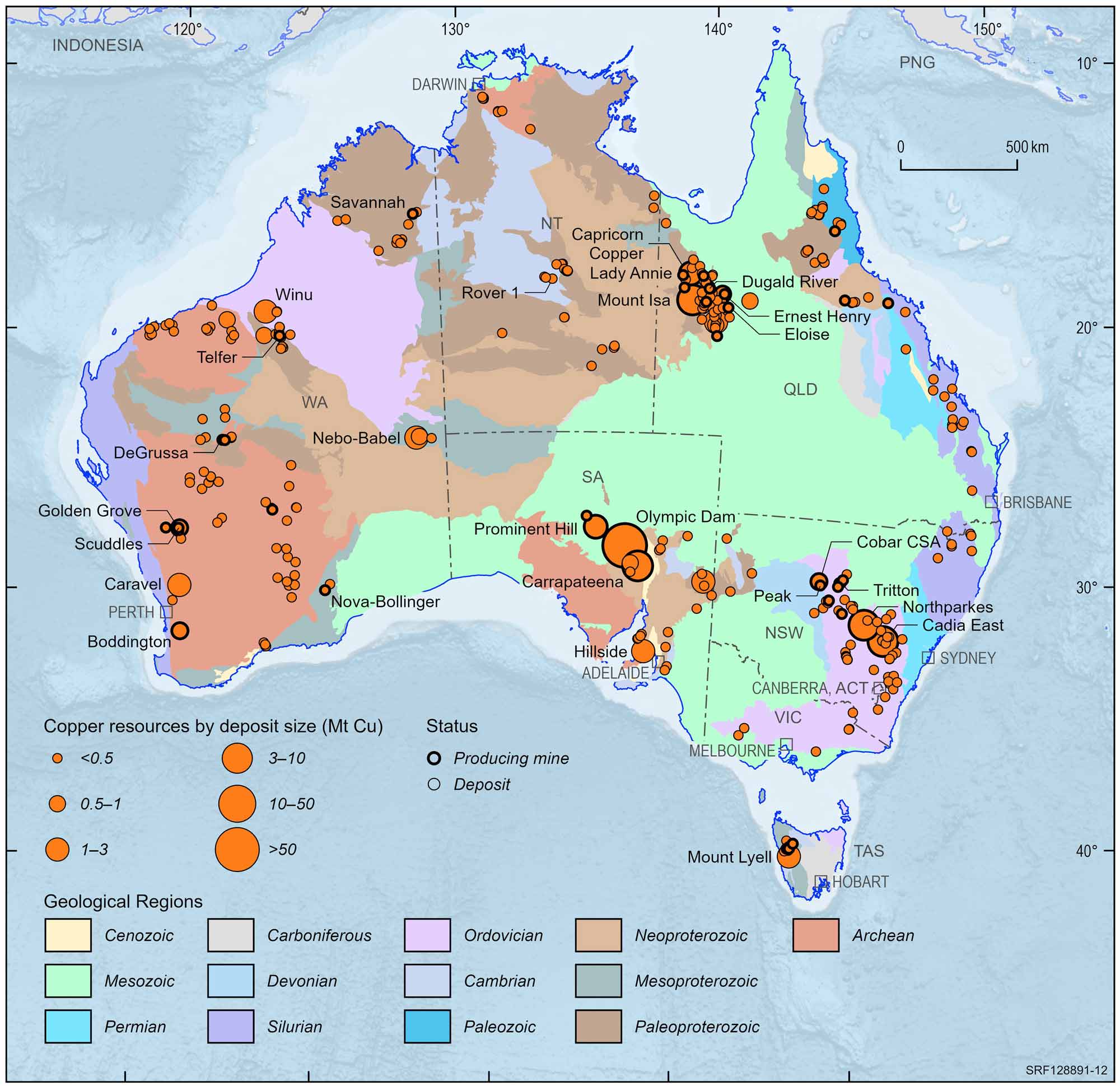
Australia holds a substantial portion of the world's copper and was ranked 2nd behind Chile in 2016, according to the United States Geological Survey (USGS). We have several copper mines which are of world significance, including the Mt Isa copper-lead-zinc deposit in Queensland and the Olympic Dam copper-uranium-gold deposit in South Australia which is mining out one of the largest copper-bearing deposits in the world. Other examples of important copper resources are at the Prominent Hill and Carrapateena copper-gold deposits in South Australia, Northparkes copper-gold, CSA copper-lead-zinc and Girilambone copper deposits in New South Wales, the Ernest Henry, Osborne and Mammoth copper deposits and copper-gold deposits at Selwyn in Queensland and copper-zinc deposits at Golden Grove and the Nifty copper deposit in Western Australia.
Australian copper deposits and operating mines, 2022.
Deposit size is based on total resources (EDR + Subeconomic Demonstrated Resources + Inferred).
For clarity, only major or significant deposits are labelled.
Mining
Although large copper deposits are mined by open-cut methods in many of the major producing countries, most of the copper ore produced in Australia comes from underground mines. The traditional method used at most mines involves the ore being broken and brought to the surface for crushing. The ore is then ground finely before the copper-bearing sulfide minerals are concentrated by a flotation process which separates the grains of ore mineral from the waste material, or gangue. Depending on the type of copper bearing minerals in the ore and the treatment processes used, the concentrate typically contains between 25 and 30% copper, however may be as high as approximately 60% copper. The concentrate is then processed in a smelter.
Processing
At some Australian mines, the copper is leached from the ore to produce a copper-rich solution which is later treated to recover the copper metal. The ore is first broken and set out on leach pads where it is dissolved by a sulfuric acid solution to leach out the copper. The copper-rich solution is then pumped to the solvent extraction plant to separate the copper as a copper complex. This is concentrated and the solution is passed to the electrowinning plant to recover the copper. The copper cathodes produced by electrowinning contain 99.99% copper which is suitable for electrical uses. This entire process is known as solvent extraction electrowinning (SX-EW).
Various methods of smelting are used to convert the concentrates to copper metal. One method is to melt them with fluxes in a smelter furnace to produce copper matte, which is a mixture mainly of iron and copper sulfides usually containing 50 to 70% copper. The molten matte is poured into a converter, which contains more fluxes and converted into blister copper, which is about 98 to 99% pure. The blister copper is tapped, further refined in an anode furnace and finally electrolytically refined to pure cathode copper.
At Olympic Dam the concentrate is flash-smelted directly to blister copper. In this process copper concentrate is fed into the smelter with oxygen-enriched air. The fine concentrate reacts or 'flashes' instantaneously as the sulfur fraction of the copper sulfides is burnt and becomes sulfur dioxide gas. Molten copper and slag fall to the hearth of the smelter. The slag forms a layer on the surface of the molten blister copper. The blister copper is removed periodically for further purifying in an anode furnace and electrolytically refined.