Gold
Page last updated:17 May 2018
Introduction
Gold is a very rare substance making up only ~3 parts per billion of the Earth's outer layer (imagine 1 billion smarties in one place and only 3 of them were made of gold!). Its rarity and physical properties have made gold one of the most prized of the Earth's natural resources.
When gold is found in rocks it is almost always in a pure state. Large pieces of gold are called nuggets and tiny pieces are known as gold dust. The most common unit of measurement for gold is the troy ounce. One troy ounce of gold weighs 31.1 grams.
Even today, achievements are rewarded by gold medals and we associate the word gold with greatness - as in 'golden rules' or 'good as gold'. Gold has always been and still is a very important metal.
Properties
Gold, like iron, copper, lead and tin is a metal. Gold is the only yellow metal and is chemically very stable. It does not readily combine with other substances and, therefore, does not corrode or tarnish. Because of this property, it is almost always found in nature as pure gold. This is referred to as 'native gold'. This meant that the early humans could collect the gold and use it without having to smelt or refine the metal from a mineral.
Gold is heavy - it weighs over nineteen times more than water and is almost twice as heavy as lead. If you had enough gold to fill a one litre milk carton, it would weigh 19.3 kilograms. The same volume of milk weighs only one kilogram.
Gold is quite soft. It is slightly harder than a fingernail but not as hard as a coin or glass. Gold, like most metals, can be hammered into thin sheets (malleable) or drawn out into thin wires (ductile). This has made gold sought after for a wide range of applications, like jewellery and in electronics. 'Gold leaf', for example, is gold that has been beaten into a sheet less than one tenth of a millimetre thick. It is then used for lettering on honour rolls in schools, or for putting gold onto picture frames and ornaments. One ounce (31 grams) of gold can be beaten into a see-through thin sheet of nine square metres or drawn out into a wire 80 kilometres long!
The Properties of Gold
| Chemical symbol | Au (from Latin: aurum) |
|---|---|
| Ore | Usually found as a native metal |
| Relative density | 19.3 g/cm3 |
| Hardness | 2.5-3 on Mohs scale |
| Malleability | High |
| Ductility | High |
| Melting point | 1064°C |
| Boiling point | 2970°C |
Uses
| Use | Description |
|---|---|
Money | Gold has been used as coins since early times, but very few coins are made from gold today. More than half the world's gold is stored by governments and banks. Institutions and individuals also use gold as a store of wealth. Check the gold price today. |
Jewellery | Although pure gold is rarely used as it is too soft, gold is often mixed (alloyed) with other metals. The term 'carat' is used to describe the purity of gold and is based on a total of 24 parts. Pure gold (100% gold and nothing else) is known as 24 carat. In 18 carat gold, for example, 18 of the 24 parts are gold and the remaining 6 parts are another metal, such as silver, nickel or copper. White gold is an alloy of gold with silver, palladium, nickel and copper. Yellow, green and red golds are produced by alloying gold with copper and silver in different proportions. |
Decoration | As gold can be rolled very thin and is durable, it's often used to coat metal or glass objects. Small sheets of gold leaf are also often used for decorative letters, gilding book edges and picture frames and to coat religious statues. |
Electronics | As it conducts electricity and is ductile, gold is used for wiring in computers - from mobile phones to rocket launchers. Gold is very useful for wiring that is difficult to repair, such as under water and in outer space, because it does not corrode or wear out quickly. An important and growing use is in the mechanism and circuitry of safety air bags in motor vehicles. |
Shielding | Rays of light do not easily pass through gold so it is useful as a protective shield against heat and light. Buildings in tropical countries sometimes have a transparent film of gold on the windows. Jet engines, space suits and space craft are often coated in gold to reduce heat and glare. Because of gold's heat reflecting properties it was used as a film coating on the Apollo 14 lunar module, the vehicle which first landed man on the moon. |
Health | Gold is used to replace or repair teeth and in the treatment of arthritis and other diseases. Lasers in industry and medicine use gold-coated reflectors to focus light energy. |
History
Gold has a very special place in human history. It has been treasured since ancient times and was the first metal used by humans, with simple gold ornaments among the earliest known metal objects. In ancient times, alchemists would spend their entire lives trying to turn other metals into precious gold. In 5000 BC, the Egyptians found gold in the bed of the Nile River and for thousands of years used gold for objects of adornment. When King Tutankhamen died, his mummified body was partly covered with gold which was still shiny when it was discovered by archaeologists over 3000 years later!
Gold has featured in many myths and legends. King Midas, King Solomon, and Jason and the Argonauts were all legendary gold seekers. Even fairy tales often mention golden objects such as eggs or harps, and most people have heard of the golden pot at the end of the rainbow.
Christopher Columbus was in search of gold when he discovered America in the 15th century.
Gold had a significant historical role in Australia. In 1823 James McBrien found traces of gold near Bathurst, NSW. However, early discoveries of gold in Australia were hushed up by the authorities for fear that all the convicts, soldiers and public servants would stop work to hunt for their fortune. In 1841, the Rev. WB Clarke found a gold nugget near Cox's River in the Blue Mountains, NSW. When he showed the gold to Governor Gipps, the Governor said, "Put it away, Mr Clarke or we shall all have our throats cut!". It wasn't until ten years later, in 1851, that Edward Hargraves (who had just returned from the gold fields in California) and his colleagues found gold near Bathurst. This time the find was publicised and within a month a thousand men were looking for gold. The area was called Ophir, after the biblical story about King Solomon's gold city.
The Bathurst gold rush was followed by discoveries in Victoria. Gold fever drew tens of thousands of immigrants from many parts of the world to the Australian colonies. Ballarat and Bendigo in Victoria became major gold sites. In 1854, gold miners on the Ballarat diggings were angry at the unfair miner's licence system. They fought against troopers in the famous Eureka Stockade battle, the only armed rebellion in Australia's history. In the early 1890s, great finds were made at Coolgardie and Kalgoorlie in Western Australia. Within 10 years of the gold rushes to Bathurst, Ballarat and Bendigo, Australia's population trebled to more than one million people. Gold discoveries spurred the development of inland towns, communications, transport and foreign trade. Although gold boosted Australia's development, its importance declined during most of the 20th century as other minerals became of greater economic significance. It underwent a resurgence in the 1980s and 1990s when the application of new technology allowed lower grade ores to be processed economically. Gold has changed where and how people live. Many towns have been developed by the wealth from mining gold and Australia also has many 'ghost towns' - when the gold supply ran out, people simply deserted the area.
The term 'digger', the nickname for Australian soldiers fighting overseas, comes from the fact that many of the World War I soldiers had literally been diggers in the goldfields just before the war.
Formation
The most common natural method of concentration of gold is through the ancient action of hot fluid inside the Earth's crust. Fluids deep in the crust are heated by the Earth's internal heat. These fluids often have moved through the rocks over a large area and 'dissolved' the gold. When these fluids cooled or reacted with other rocks the dissolved gold precipitated (came out of the fluid) in cracks or fractures forming veins. If the fluids move over a large enough area, and dissolve the gold for a long enough period of time, gold can be concentrated in amounts in the parts per thousand or even greater. In Australia this concentration of gold took place in the Earth hundreds of millions of years ago in the eastern states, and thousands of millions of years ago in Western Australia.
As well as gold, the fluids can carry other dissolved minerals, such as quartz. This is why gold is often found with quartz. These are known as primary gold deposits and to extract the gold the rock containing the veins of gold has to be dug up (mined), crushed and processed.
Some rocks containing gold veins have been exposed on the surface and are eroding away. The gold that these rocks contained has been washed down into creeks to form alluvial (placer) gold deposits. Here, the gold is further concentrated by the action of water. Because gold is heavier than most of the material moved by a creek or river, it can become concentrated in hollows and trapped in the bed of the river. These are known as secondary (alluvial) gold deposits and they can be worked using a gold pan or cradle. Alluvial gold deposits sparked the Australian gold rushes of the 1850's
Resources
Mostly, gold is spread throughout the rocks and soil around us but in such low amounts that it's not worthwhile trying to get it out. However, there are some places where there is enough gold to make it economic to mine. Most gold mined in Australia today cannot be seen in the rock, it is very fine grained and mostly has a concentration of less than 5 grams in every tonne of rock mined. The feasibility of mining low concentrations of gold largely depends on the price of gold. Gold is bought and sold every day on international gold markets. The price fluctuates according to demand by buyers and the amount being sold by sellers.
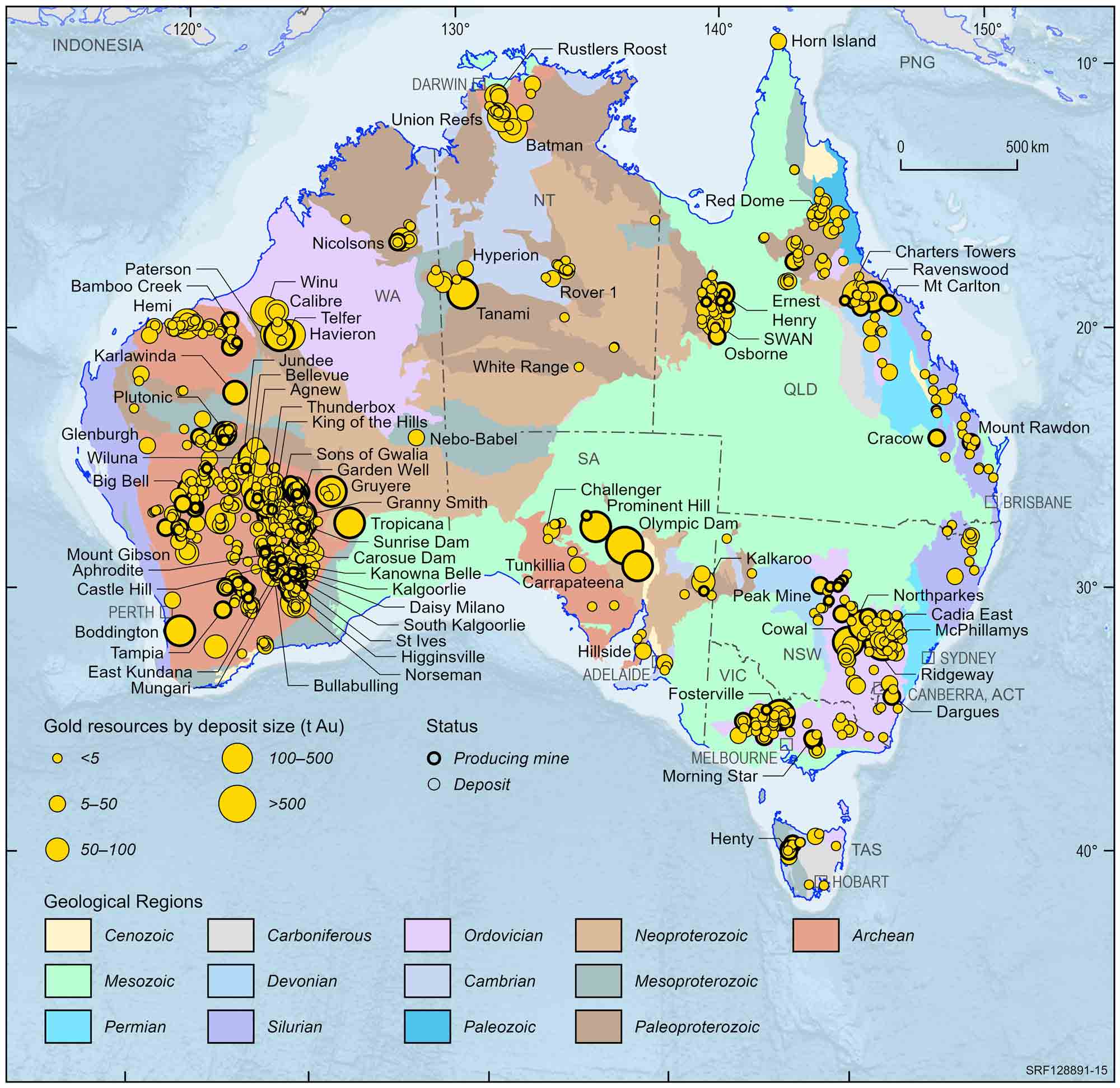
Australian gold deposits and operating mines, 2022.
Deposit size is based on total resources (EDR + Subeconomic Demonstrated Resources + Inferred).
For clarity, only major or significant deposits are labelled.
In a few places gold is sufficiently concentrated in the rocks for it to be worth mining. Australia (especially Western Australia) is the one of the world's top producers of gold. About 60% of Australia's gold resources occur in Western Australia, with the remainder in all other States and the Northern Territory. Virtually all resources occur in primary deposits, many of which have undergone some degree of weathering. Weathered primary deposits are important to the gold industry because they are usually easier and cheaper to mine and the gold is easier to recover.
Examples of primary deposits include those mined at Kalgoorlie in the Super Pit, Granny Smith, St Ives, Norseman and Mount Magnet (WA), Gympie and Ravenswood (Qld), Callie (NT), Stawell (Vic), Cadia (NSW), Henty (Tas) and Challenger (SA). At Olympic Dam (SA) gold occurs and is mined with copper and uranium. Secondary deposits are no longer major sources of gold in Australia.
Gold usually occurs in its metallic state, commonly associated with sulphide minerals such as pyrite, but it does not form a separate sulphide mineral itself. The only economically important occurrence of gold in chemical combination is with tellurium as telluride minerals.
The largest gold nugget ever found was the 'Welcome Stranger', found in 1869 just under the soil at the base of a tree! It weighed 70 kg and on today's value would be worth over $3 million. Quite a find! The second largest gold nugget ever found was the Welcome Nugget (68.98 kg), that was discovered by a group of twenty-two Cornish miners at the Red Hill Mining Company site at Bakery Hill in Ballarat, Victoria, Australia, on June 9, 1858. It was located in the roof of a tunnel 55 metres underground.
Mining
There are both open-cut and underground gold mines in Australia but most of Australia's gold production comes from open-cut mines. Earth-moving equipment is used to remove waste rock from above the ore body and then to mine the ore. Waste and ore are blasted to break them into sizes suitable for handling and transport to waste dumps or, in the case of the ore, to the crusher.
Underground mining is used where the depth of ore below the surface makes open-cut mining uneconomic. Vertical shafts and declines (spiral tunnels) are used to move people and equipment into and out of the mine, to provide ventilation and for hauling the waste rock and ore to the surface. Extensions of deposits mined by open pit methods may be mined later by underground methods beneath the old open pit.
Processing
The processing of gold ore involves crushing, treatment with chemicals, melting (smelting) and further purification. It is then poured into moulds where it cools and hardens as gold bars called 'bullion', which make the gold easy to stack and transport.
The first stage of processing gold ore is crushing. The gold then needs to be separated from the resultant powder. Coarse gold may be removed by gravity concentration. The powder is mixed with water, the gold sinks and the other wastes are washed away. Fine gold in crushed ore will be processed differently depending on the nature of the gold ore itself.
Free-milling ore is the name for when gold can be recovered by crushing, grinding and cyanidation (treatment with a dilute cyanide solution) without additional processing. In refractory ore the gold is locked in sulphide minerals, so to achieve satisfactory levels of gold recovery additional processing is required before cyanidation. Sulphide minerals in refractory ores are converted to oxides by either roasting or biological leaching to release the gold. In biological leaching the oxidation is caused by the action of specific bacteria on the ore. The tonnage of refractory ore to be roasted or leached is greatly reduced by first producing a finely ground concentrate.
Ground ore or treated concentrate is placed in a weak solution of sodium cyanide, which dissolves gold and forms a slurry of gold-bearing solution and other solids. Some ores may be treated by heap-leaching. This involves sprinkling a weak cyanide solution over an open pile of ore stacked on an impervious base. The solution percolates through the ore, leaching gold as it goes and is drawn off at the base before being treated to recover the gold.
In both cases, the gold is recovered from the gold-bearing solution in a process in which pellets of activated carbon made from charred coconut husks are added to the slurry and the gold-bearing ions are adsorbed onto the pellet surface. The pellet load is moved through a number of linked tanks containing slurry in a direction opposite to the slurry movement. The pellets loaded with gold are removed and the gold is stripped from them by washing in a solution of hot cyanide. The carbon used in the process is recycled and an electric current is passed through the new solution, depositing the gold on a steel wool cathode. The gold laden cathode is treated with hydrochloric acid to dissolve any residual steel and the gold sludge is filtered and dried, ready for smelting. At this stage the gold-bearing material may also contain silver and base metals.
Gold is smelted in a crucible furnace to produce unrefined bullion. In smelting, base metal impurities are oxidised and absorbed, leaving the precious metals to be poured into ingot moulds. Smelted gold is then refined. Several refining processes are used in Australia - chlorination, electrolytic and aqua regia.
In the chlorination process (Miller process), chlorine is introduced to melted bullion in a crucible furnace. The gas reacts with silver and any remaining base metals to form chlorides, bubbles of which rise to the surface of the molten bullion and are removed. The molten, refined gold is then cast into bars. The electrolytic process (Wohlwill process) involves dissolving gold from the bullion (anode) in a chloride solution and redepositing the gold on a pure gold or titanium cathode. Silver remains on the anode. The cathodes are melted and cast. In the third process the unrefined bullion is dissolved in aqua regia and silver is precipitated as silver chloride. Sulphur dioxide gas is passed through the remaining solution and gold is precipitated as a fine metallic powder. The gold is then melted and cast.