Iron
Page last updated:14 May 2025
Introduction
Iron is the backbone of the world we have built around us and it is the basic ingredient of steel (iron plus carbon). Iron is a very useful metal because it can be mixed with other metals to make a whole variety of 'alloys' which are even stronger and don't rust easily and can be shaped into products from cars to pins, household appliances to buildings, bridges to railways, food cans to tools. In short, we rely on iron (as steel) to make almost everything we need for living in the 21st century. Today we use twenty times more iron (in the form of steel) than all other metals put together.
Iron (Fe) is one of the most abundant rock-forming elements, constituting about 5% of the Earth's crust. It is the fourth most abundant element after oxygen, silicon and aluminium and, after aluminium, the most abundant and widely distributed metal. The Earth's magnetic field is due to the iron (and nickel) in its core, so when we use a compass we are making use of the iron underneath us. Iron is a silver-grey metal that quickly rusts when exposed to air and water. It is responsible for the red colour in many of our rocks and the deep red sands of the Australian deserts. Iron is not usually found in pure form within rocks, instead it is combined with oxygen in ore minerals such as hematite (from the Greek word meaning 'blood-stone').
Properties
In the Earth's crust iron is found mainly as minerals of iron oxide such as hematite, magnetite, goethite and limonite. The minerals that are mostly used as ore for making iron are hematite (Fe2O3) and magnetite (Fe3O4). Iron is quite soft and easily worked, but it has a very high melting point of 1538°C. Iron and some alloys of iron are also magnetic.
Iron is about eight times heavier than water (its relative density is 7.87). When iron is exposed to air it starts to turn back into iron oxide and the red powder that forms on the surface of iron is what we call rust. You may have seen rust on old cars or old iron sheds. To make iron stronger and less likely to rust it can be combined with carbon and other elements to make steel. Steel is one of the world's most recycled products, with about 60% of steel available for recycling going back into making new steel.
The Properties of Iron
| Chemical symbol | Fe, comes from the Latin word for iron ferrum |
|---|---|
| Ore | Iron oxides e.g. hematite and magnetite |
| Relative density | 7.87 g/cm3 |
| Hardness | 4 on Mohs scale |
| Malleability | High |
| Ductility | High |
| Melting point | 1538°C |
| Boiling point | 2862°C |
Uses
About 98% of world iron ore production is used to make iron in the form of steel. Iron in cast form has many specific uses (e.g. pipes, fittings, engine blocks) but pure iron is quite soft. Adding a small amount of carbon (usually less than 1%) makes iron into steel which is significantly harder and extremely versatile. There are many different kinds of steel made by adding carbon along with other elements such as chromium, manganese, nickel, molybdenum to form a range of alloys with different properties (e.g. stainless steel). By changing the proportions of these additional elements, it is possible to make steels suitable for a great variety of uses. The table below shows the special properties and uses of some iron compounds.
| Name of iron compound | main components | properties | uses |
|---|---|---|---|
| Cast iron | iron + up to 5% carbon Sometimes 1-3% silicon | rusts easily hard brittle | camp ovens engine cylinder blocks woks |
| Galvanised iron | iron + zinc coating | resists rusting malleable | roofing motor vehicle bodies boats |
| Tin plate | iron + tin coating | resists rusting | tin cans for food preservation |
| Steel | iron + less than 1% carbon | hard strong malleable | buildings machinery transportation cans & containers household appliances |
| Stainless steel | iron + carbon nickel chromium | doesn't rust malleable | cutlery hospital equipment motor vehicle parts |
| Tool steel | iron + carbon vanadium, chromium tungsten and/or molybdenum | very hard very brittle | metal cutting tools drill bits |
Steel's desirable properties and its relatively low cost make it the main structural metal in engineering and building projects, accounting for about 90% of all metal used each year. About 60% of iron and steel products are used in transportation and construction, 20% in machinery manufacture, with the remainder in cans and containers (in the oil and gas industries) and in various appliances and other equipment, see table below for further uses of iron and steel.
| Use | Description |
|---|---|
| Transportation | Steel railway carriages/engines, ships, car frames, engine cylinders. |
| Construction | Steel buildings, bridges (such as the Sydney Harbour Bridge), reinforcing in concrete buildings, roofing, cladding, doors, fencing. |
| Machinery | Steel engines, pumps, cranes, workshop equipment (e.g. cutting tools, drill bits). |
| Wire products | Steel wire fences, ships' cables, staples, door screens, nuts & bolts. |
| Storage | Steel food containers, storage tanks. |
| Oil and gas | Steel drill rods, casing, pipelines. |
| Appliances and equipment | Steel refrigerators, washing machines, dishwashers, cutlery, hospital equipment. |
| Health | Pure iron is needed for proper plant growth. Animals need iron for making energy and carrying blood around the body (foods rich in iron include red meat and liver, egg yolks and leafy green vegetables). Iron was the first element to be recognised as essential for people. A physician in 1681 successfully used iron to treat patients who were pale, lacking in energy and suffering from anaemia. Iron chloride is used in water treatment and purification. |
| Fun | Iron filings are used in 'sparklers'. |
| Electronics | Iron chloride is used to etch copper in the making of electrical printed circuits. |
| Cooking | Cast iron camp ovens and woks. |
| Decoration | Wrought iron outdoor furniture, porch railings and other decorative items. |
History
Iron is among the oldest metals known to humans. Paleolithic Man used finely ground haematite as body paint. Around 4000 BC, the Egyptians and Sumerians first used iron from meteorites to make beads, ornaments, weapons and tools. The time line of Iron Age varied geographically; for instance the Hittites forged iron (they heated it, then hammered it, then cooled it quickly to produce iron that was harder than the bronze that people had been using before) around the period of 1300 - 1100 BC and similarly according to Tewari (2003), archaeological evidence indicates iron working in India occurred around 1800 to 1000 BC. By the time of the Roman Empire, iron was being used for beds, gates, chariots, nails, saws, axes, spears, fishhooks and tools for sharpening. During the Middle Ages, with the introduction of the iron cannon and cannon ball, the consumption of iron increased to overtake copper and bronze as the most widely used metal. In the late 19th century the Age of Steel began, with wooden ships giving way to steel, machinery coming to factories and the invention of the railroad. Iron is indispensable to modern civilisation and people have been skilled in its use for more than 3,000 years. However, its use only became widespread in the 14th century, when smelting furnaces (the forerunner of blast furnaces) began to replace forges.
Formation
Iron ores are rocks from which metallic iron can be economically extracted. Most deposits of iron ore in the world are found in rocks known as banded iron formations (BIFs). These are sedimentary rocks that have alternating layers of iron-rich minerals and a fine-grained silica rock called chert.
Many of the banded iron formations that are being mined today were formed millions of years ago. About 3000 million years ago there was no or very little oxygen dissolved in the oceans. However, the oceans did contain a lot of dissolved silica, which came from the weathering of rocks. Every now and again this silica precipitated out from the seawater as layers of silica jelly, which slowly hardened to become the rock we call chert. Soluble iron oxide was also produced from the weathering of rocks and was also washed into the sea by rivers.
About 2500 million years ago the oceans were inhabited by bacteria that developed the ability to photosynthesise and produce oxygen. There were seasonal 'blooms' that released huge amounts of oxygen into the seawater that reacted with the soluble iron oxide to form insoluble iron oxide. This precipitated out of solution as the minerals magnetite and hematite forming layers of iron among the other layers of sediment on the sea floor.
Over many millions of years these processes of precipitating silica and iron oxide were repeated over and over again resulting in the deposition of alternating layers of chert, hematite and magnetite. The name banded iron formation comes from the characteristic colour banding of these huge deposits. The process continued for nearly a billion years and eventually let to the accumulation of oxygen in the atmosphere.
Resources
Most of the world's important iron ore resources occur in banded iron formations, which are almost exclusively of Precambrian age (i.e. greater than 541 million years old). BIFs occur on all continents.
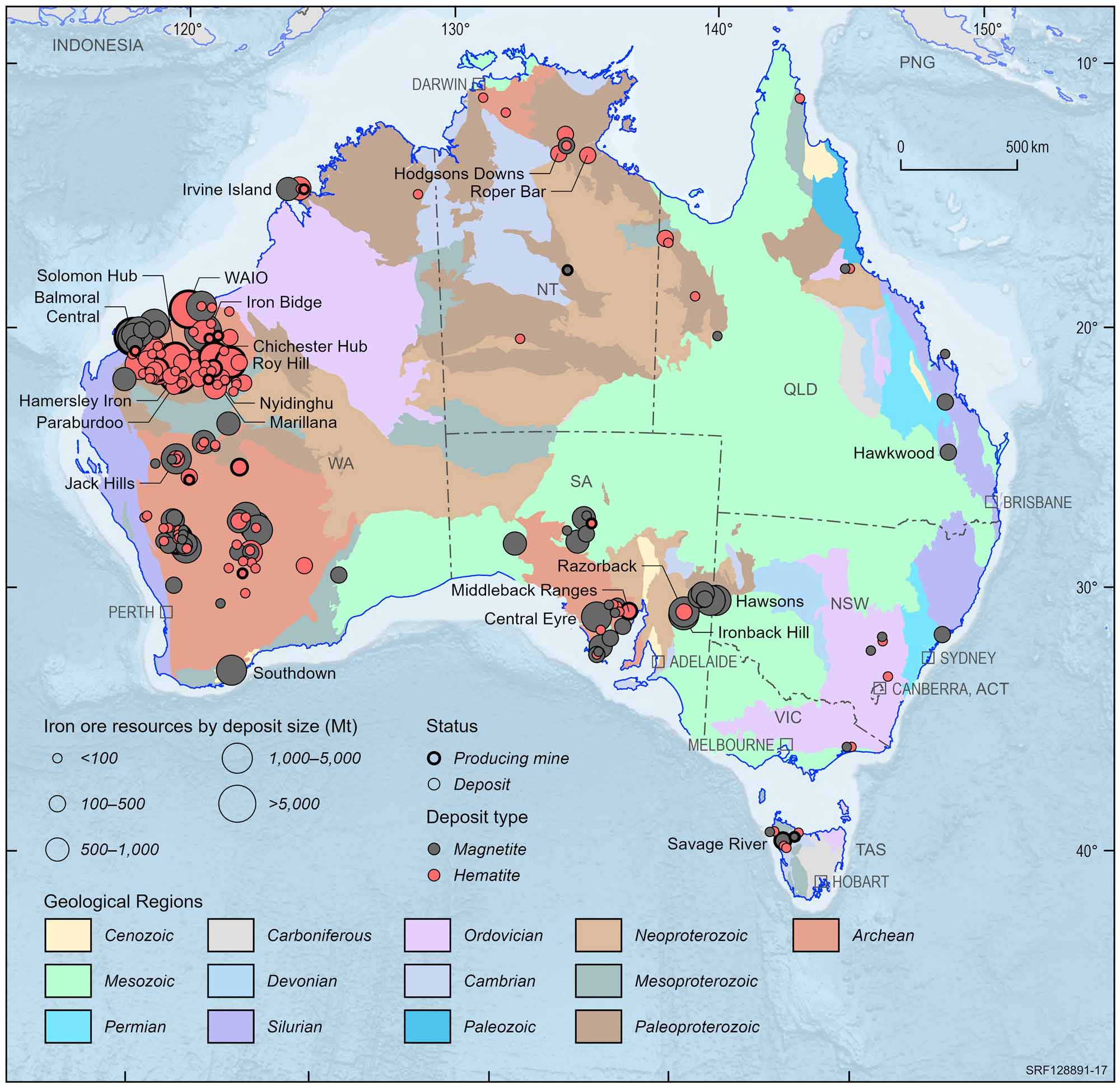
Iron was the first metal to be discovered in Australia by explorer Edward John Eyre in the Middleback Ranges in South Australia. Although iron ore resources occur in all the Australian States and Territories, almost 90% of identified resources occur in Western Australia, including almost 80% in the Hamersley Province, one of the world's major iron ore producing regions. Australia is one of the largest producers of iron ore in the world and iron ore is the foundation for one of Australia's major export industries.
Australian iron ore deposits and operating mines, 2022.
Deposit size is based on total resources (EDR + Subeconomic Demonstrated Resources + Inferred).
For clarity, only major or significant deposits are labelled.
In the Hamersley Ranges in the Pilbara district of Western Australia there are three main types of iron deposit: iron oxide enrichments within BIFs e.g. Mt Tom Price; iron oxides deposited along ancient, mainly Tertiary age river channels (palaeochannels); and iron oxide deposits formed from the erosion of existing orebodies (detrital iron ore deposits).
The BIF enrichment deposits comprising hematite and hematite goethite are the most important in regard to resources and production. The iron content of these ores varies widely and until recently most deposits needed to have an average grade of more than 60% iron for mining to be commercially viable. However, some deposits can now have iron grades between 56%-59% iron and be commercially viable. BIF enrichment deposits occur in Western Australia in the Pilbara (e.g. Yarrie), and the Yilgarn Block (e.g. Koolyanobbing) and in South Australia (e.g. Iron Duke, Middleback Range). The palaeochannel deposits composed of pisolitic limonite are the next in importance and are prized for their low impurities such as phosphorus. They are not as rich in iron as the BIF enrichment ores. Those mined usually contain 57%-59% iron. Detrital iron ore deposits are found downhill of the BIF enrichment deposits from which they have been eroded. They are usually easily recovered and have a grade of between 40%-55% iron.
Mining
Most iron ores mined today comprise the iron oxide minerals hematite, Fe2O3 (70% Fe); goethite, Fe2O3s H2O, (63% Fe); limonite, a mixture of hydrated iron oxides (up to 60% Fe); and magnetite, Fe3O4 (72% Fe). As with most iron ore mines throughout the world, all the major Australian iron ore mines are open cut. The iron-ore bearing rock is first blasted and dug up from open pit mines. The ores from the major mines in Western Australia's Pilbara region are hauled from working faces to crushing and screening plants using trucks that can carry over 300 tonnes. There are three major Pilbara iron ore producers: BHP Billiton Ltd (BHP), Rio Tinto Ltd (Rio) and Fortescue Metals Group Ltd (FMG).
Processing
Hematite and magnetite ore processing includes crushing, screening and grinding to produce hematite lumps and fines. Magnetite ore is further processed through magnetic separation, an important process in producing magnetite iron concentrate.
Concentration includes all the processes that will increase (upgrade) the iron content of an ore by removing impurities. Beneficiation, a slightly broader term, includes these processes as well as those that make an ore more usable by improving its physical properties (e.g. pelletising and sintering). Many of the iron ore mines employ some form of beneficiation to improve the grade and properties of their products. At many operating mines, including Mount Tom Price, Paraburdoo, Mount Whaleback and Christmas Creek, ore processing facilities have been constructed to enable beneficiation of low-grade iron ores, including ores which have been contaminated with shale, to be mined and, after upgrading, sold as high-grade products.
Pelletising is a treatment process used for very fine or powdery ores. Pellets are an ideal blast furnace feed because they are hard and of regular size and shape. In Australia, concentrates pumped from Savage River are pelletised at Port Latta for shipment to domestic and overseas markets and fine Middleback Range ores are pelletised prior to smelting in the Whyalla blast furnace.
Sintering is a process used to agglomerate iron ore fines in preparation for blast-furnace smelting and is usually carried out at iron and steelmaking centres. It involves the incorporation of crushed limestone, coke and other additives available from iron and steelmaking operations. These additives include wastes extracted from furnace exhaust gases, scale produced during rolling mill operations and coke fines produced during coke screening.
Pig iron is an intermediate step in the production of steel and is produced by smelting iron ore (commonly in lump, pellet or sinter form) in blast furnaces. Blast furnaces in Australia are located at Port Kembla and Whyalla. The removal, by oxidation, of impurities in pig iron such as silicon, phosphorus and sulfur and the reduction in the carbon content, results in the production of steel.
Adding metals like nickel, chromium, manganese or titanium gives steel special properties like electrical resistance and resistance to wear, rust, impact, shock or expansion when heated.The cooled steel is shaped and can be coated with tin, zinc or paint to help protect it from rusting, creating products such as Zincalume and Colorbond.
References
The Age of Iron, Chapter 5.
Tewari, Rakesh (2003). "The origins of Iron Working in India: New evidence from the Central Ganga plain and the Eastern Vindhyas" (PDF). Antiquity. 77: 536—545.